Multi-claw cationic functional monomer
A technology of methyl and butenyl ether, which is applied in the field of multi-claw cationic functional monomers, can solve problems such as difficulty in use and slow dissolution speed, and achieve the effects of less scum generation, low moisture content, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
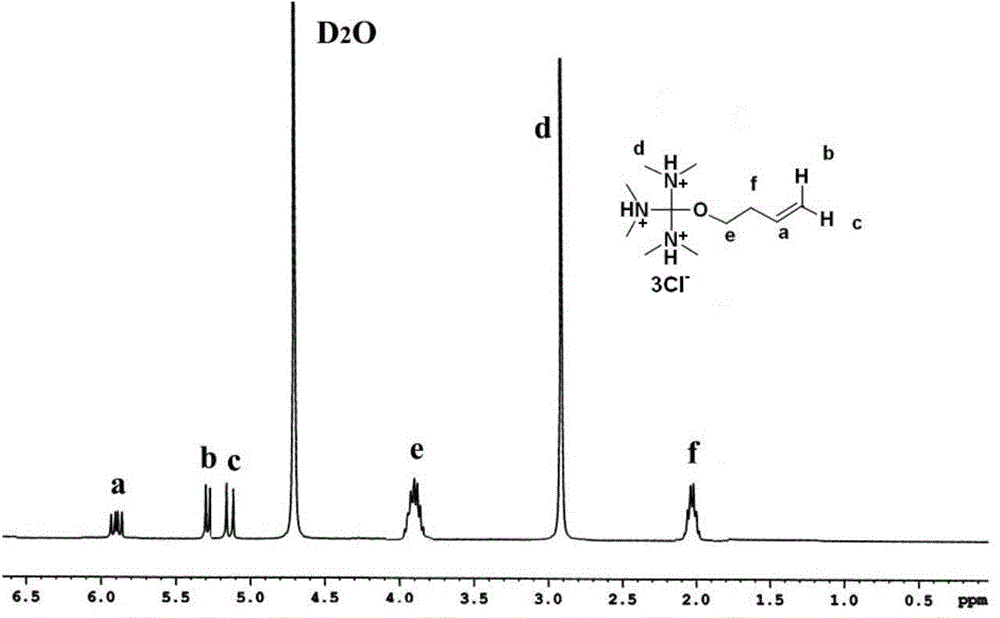
[0042] Example 1. Preparation of polyclaw cationic functional monomer tris(dimethylamino)methyl-3-butenyl ether hydrochloride
[0043] (1) Synthesis of hexamethylproguanil
[0044] ① Synthesis of dimethylamino-N,N-dimethylchloroalkenimium chloride (C5H12Cl2N2, 171.07g / mol)
[0045] The reaction equation is as follows:
[0046]
[0047] Second, add 1mol of tetramethylurea (Sinopharm Chemical Reagent Co., Ltd., C 5 H 12 N 2 O, 116.16g / mol), 200mL of chloroform (Jinan Reagent Factory), under nitrogen protection, 2.1mol of oxalyl chloride (Sinopharm Chemical Reagent Co., Ltd., C 2 H 2 Cl 2 , 126.93g / mol), stirred, heated to reflux in an oil bath, cooled to room temperature after 4 hours of reaction, distilled under reduced pressure to distill out the concentrate, added 200 mL of anhydrous ether (Tianjin Bodi Chemical Co., Ltd.), cooled to 0 °C, The solid product obtained by suction filtration was washed with anhydrous ether for several times, and dried in vacuum for 24 h...
Embodiment 2
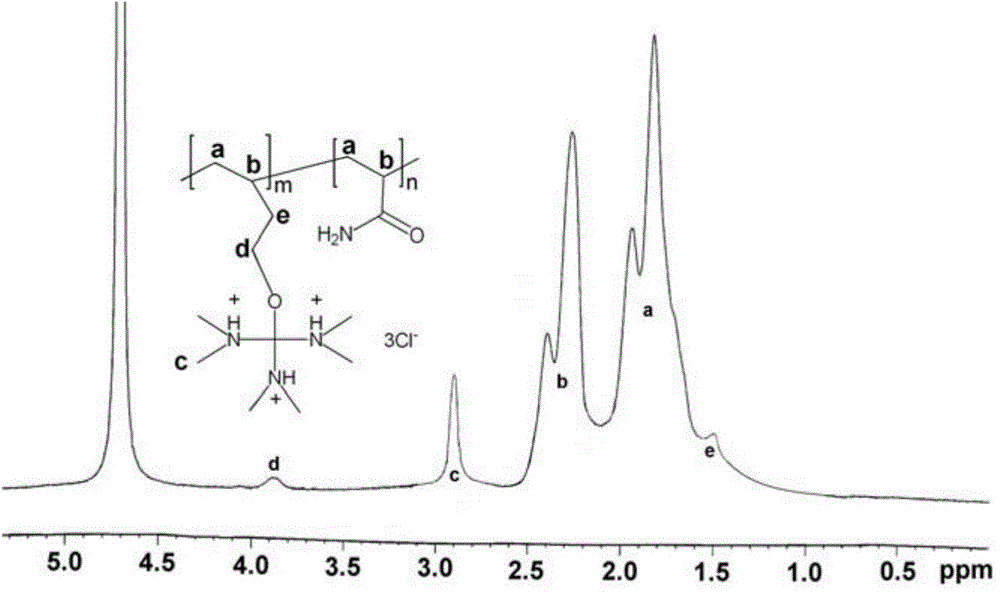
[0062] Example 2. Preparation of cationic polymer MCF-1 using monomer tris(dimethylamino)methyl-3-butenyl ether hydrochloride
[0063] The reaction equation is as follows:
[0064]
[0065] 22g of acrylamide (Sinopharm Chemical Reagent Co., Ltd.) (C 3 H 5 NO, 71.08g / mol) and 18g tris(dimethylamino)methyl-3-butenyl ether hydrochloride monomer were dissolved in 50mL deionized water to obtain an aqueous monomer solution, and then the monomer aqueous solution was added to a solution containing 8.25g Span-80 (Jiangsu Haian Petrochemical Plant) and 6.75g Tween-80 (Jiangsu Haian Petrochemical Plant) in 135g cyclohexane (Sinopharm Group Chemical Reagent Co., Ltd.), emulsified under a high shear homogenizer 15min to obtain an emulsion. Add the above-mentioned emulsion to the 500mL four-necked flask equipped with a stirrer, thermometer, dropping funnel, and ventilation tube, and after stirring for 20 min of nitrogen and oxygen removal, dropwise add 0.06g potassium persulfate (Sino...
Embodiment 3
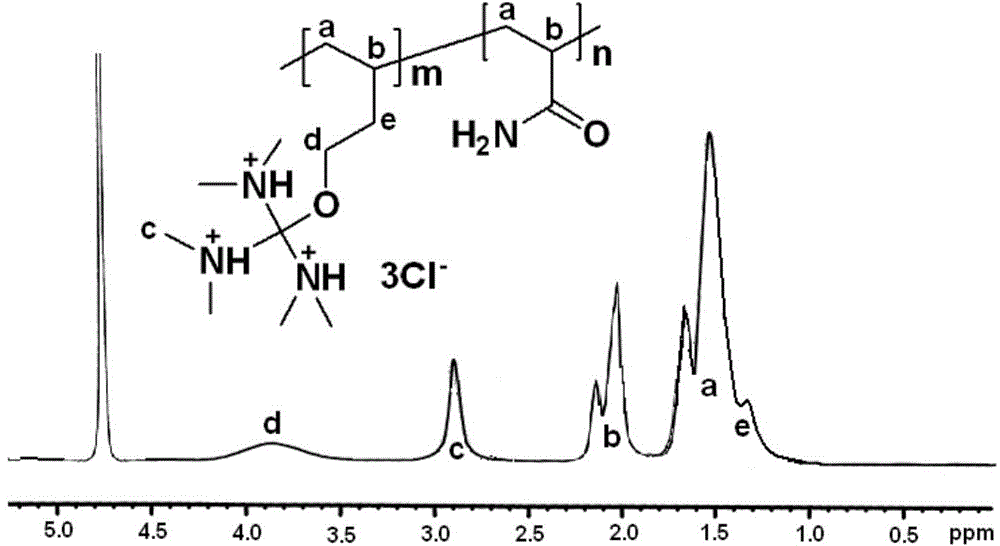
[0070] Example 3. Preparation of cationic polymer MCF-2 using monomer tris(dimethylamino)methyl-3-butenyl ether hydrochloride
[0071] Dissolve 16g acrylamide and 24g tris(dimethylamino)methyl-3-butenyl ether hydrochloride monomer in 50mL deionized water to obtain an aqueous monomer solution, and then add the monomer aqueous solution to a solution containing 8.25g Span-80 and 6.75g of Tween-80 in 135g of cyclohexane, emulsified under a high shear homogenizer for 10min to obtain an emulsion. Add the above-mentioned emulsion to the 500mL four-necked flask equipped with a stirrer, a thermometer, a dropping funnel, and a vent pipe, and after stirring for 20min of nitrogen and oxygen removal, add dropwise an aqueous solution containing 0.06g potassium persulfate and 0.02g sodium bisulfite. 10 mL, the reaction temperature was controlled to be 45 °C, and the reaction was stopped after 4 h of constant temperature. Add acetone for precipitation, filter under reduced pressure, wash twi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com