Preparation method according to D-serine through splitting method
A technology of serine and resolving agent, applied in chemical instruments and methods, preparation of cyanide reaction, preparation of organic compounds, etc., can solve crystal data without diastereomeric salts, research without energy difference of diastereomeric salts , no assumptions and other issues, to achieve the effect of simple operation, effective utilization rate improvement and consumption reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
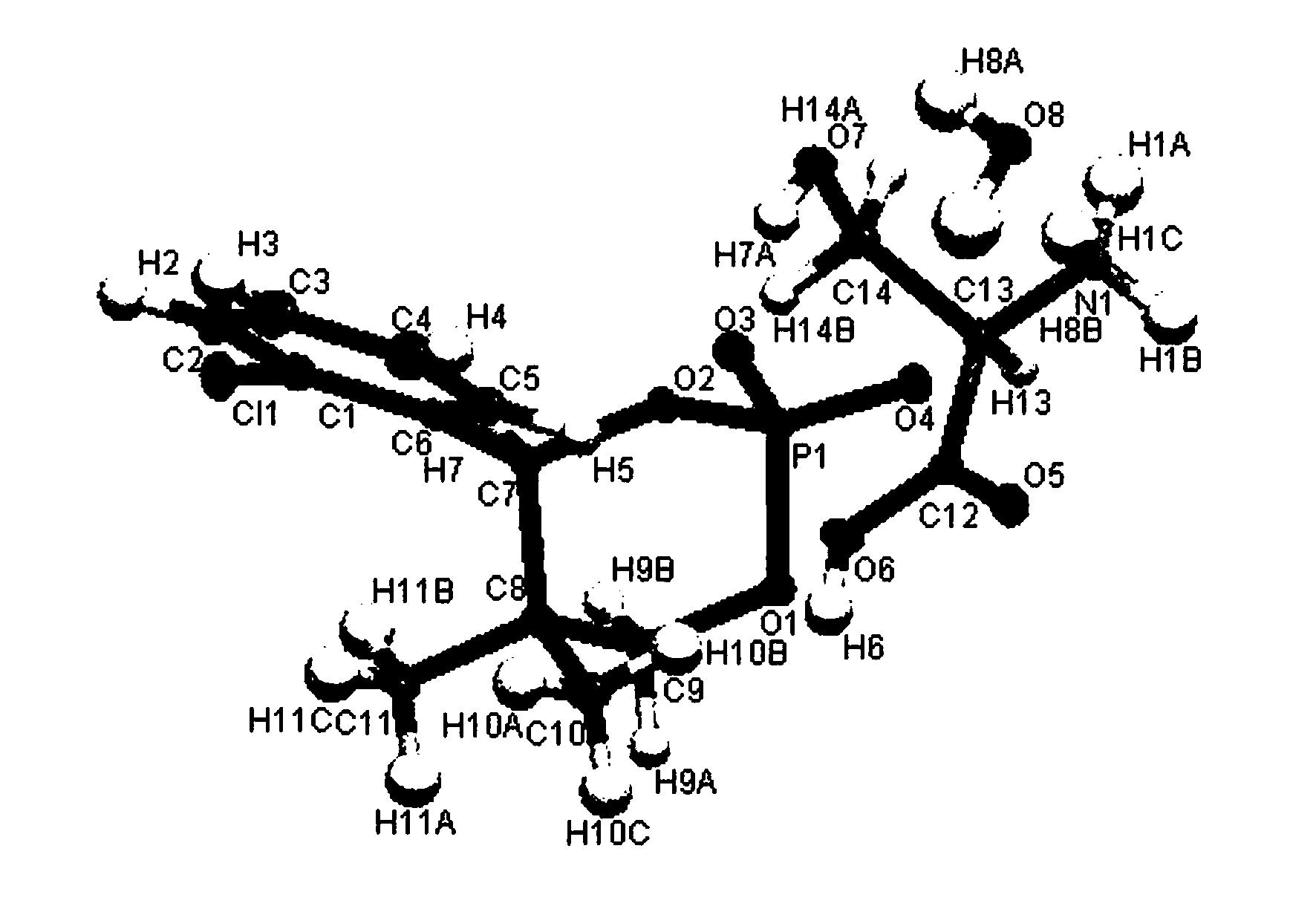
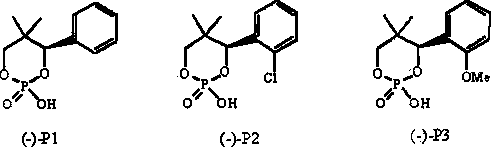
[0037] Step a: Add 34.6g (0.3mol) of phosphoric acid to 400mL of isopropanol water (volume ratio is 9 / 1), add 105.1g (1mol) of hydroserine and (-)-4-(2- Chlorophenyl)-2-hydroxyl-5,5-dimethyl-2-oxo-1,3,2-dioxaphosphorinane resolving agent 138.3g (0.5mol), heating to reflux until the solution is clear , stirred for 1h, cooled and crystallized, filtered, and washed the filter cake with 50mL of isopropanol to obtain 201.7g of a white solid. The molecular structural formula is shown in compound (1). The crystal was tested as a monohydrate, see the attached figure 1 :
[0038]
[0039] Compound (1) 。
[0040]
[0041] Step b: Dissolve the above filter cake in 200mL isopropanol / water solution (volume ratio: 20 / 1), adjust pH=5.68 with NaOH solution, filter, wash with 20mL isopropanol, and dry to obtain 48.7g of D-serine , yield 93%, -14.75°(c=10 2N HCl), ChemicalBook value: -14.75° (c=10 2N HCl). After the filtrate was concentrated, 300 mL of water was added, the pH was adj...
Embodiment 2
[0044] Step a: Add 608 g (6 mol) of 36% hydrochloric acid to 400 mL of methanol water (volume ratio 1 / 1), add 105.1 g (1 mol) of hydrochloric acid to the solution and (-)-4-(2- Chlorophenyl)-2-hydroxyl-5,5-dimethyl-2-oxo-1,3,2-dioxaphosphorinane resolving agent 138.3 g (0.5mol), heated to reflux until the solution is clear , stirred for 1 h, cooled and crystallized, filtered, and washed the filter cake with 50 mL of methanol to obtain 202.1 g of a white solid, the molecular structure of which was D-serine (-)-4-(2-chlorophenyl)-2-hydroxyl-5, 5-Dimethyl-2-oxo-1,3,2-dioxaphosphorinane double salt monohydrate.
[0045] Step b: Dissolve the above filter cake in 300mL of water, adjust the pH to strong acidity with hydrochloric acid, stir at room temperature for 3 hours, a large amount of solid precipitates, filter, and recover (-)-4-(2-chlorophenyl)-2-hydroxy-5 , 133.8 g of 5-dimethyl-2-oxo-1,3,2-dioxaphosphorinane, the recovery rate was 97%, and the recovered resolving agent was ...
Embodiment 3
[0048] Step a: Add 98 g (0.5 mol) of 50% sulfuric acid to 400 mL of ethanol, add 105.1 g (1 mol) of cycloserine and (-)-4-(2-chlorophenyl)-2-hydroxyl-5 ,5-Dimethyl-2-oxo-1,3,2-dioxaphosphorinane resolving agent 138.3g (0.5mol), heated to reflux until the solution was clear, stirred for 1h, cooled and crystallized, filtered, Wash the filter cake with 50 mL of ethanol to obtain 192.5 g of double salt filter cake white solid, the molecular structure is D-serine (-)-4-(2-chlorophenyl)-2-hydroxyl-5,5-dimethyl- 2-Oxo-1,3,2-dioxaphosphorinane double salt anhydrous.
[0049] Step b: Dissolve the above filter cake in 200mL ethanol / water solution (volume ratio is 20 / 1), adjust pH=5.68 with NaOH solution, filter, wash with 20mL ethanol, and dry to obtain 49.5g of D-serine, yield 94%. -14.73°(c=10 2N HCl), ChemicalBook value: -14.75° (c=10 2N HCl). After the filtrate is concentrated, add 300mL of water, adjust the pH to strong acidity with hydrochloric acid, stir at room temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com