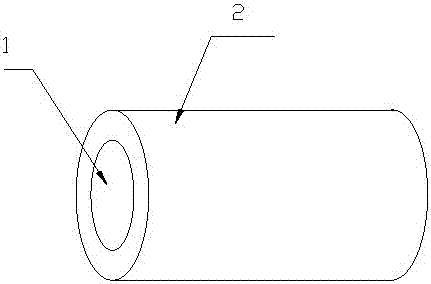
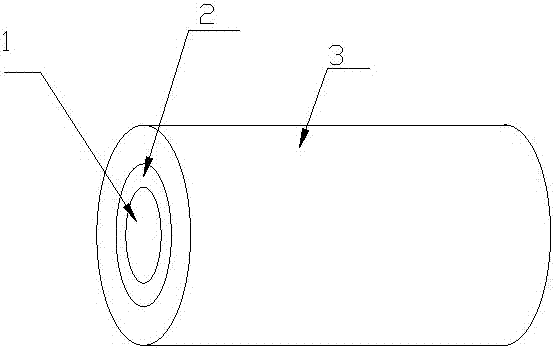
A kind of braided wire for medical use and its preparation method and product
A braided wire and medical technology, applied in the field of medical materials, can solve the problems of braided wire movement, sling or patch erosion, and insufficient softness, and achieve the effects of reducing sliding and friction, avoiding erosion and exposure, and improving bonding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Prepare the braided wire for medical use as follows:
[0058] S1. Preparation of electrospinning solution: Polylactic acid was dissolved in hexafluoroisopropanol with a mass volume concentration of 5%.
[0059] S2. Perform electrospinning. The electrospinning conditions are as follows: the electrospinning voltage is 25 kV, the propulsion speed is 0.5 mL / h, and the distance from the electrospinning head to the grounded metal plate is 30 cm. After the start of electrospinning, at a distance of about 10cm from the filament outlet, a bundle of polypropylene threads continues to pass in parallel, and the polypropylene threads are continuously conveyed from one end to the other; at the same time, the polypropylene thread keeps rotating by itself during the conveying process. The conveying speed of the polypropylene wire was 3 m / min, and the rotation speed of the polypropylene wire was 100 rpm. Electrospinning will be evenly wound onto the surface of the polypropylene thread ...
Embodiment 2
[0062] Prepare the braided wire for medical use as follows:
[0063] S1. Preparation of electrospinning solution: Polylactic acid was dissolved in hexafluoroisopropanol to make the mass volume concentration of polylactic acid 15%.
[0064] S2. Perform electrospinning. The electrospinning conditions are as follows: the electrospinning voltage is 25 kV, the propulsion speed is 5 mL / h, and the distance from the electrospinning head to the grounded metal plate is 30 cm. After the start of electrospinning, at a distance of about 20 cm from the filament outlet, a bundle of polypropylene threads continued to pass in parallel, and the polypropylene thread was continuously conveyed from one end to the other; at the same time, the polypropylene thread kept rotating itself during the conveying process. The conveying speed of the polypropylene wire was 12 m / min, and the rotation speed of the polypropylene wire was 1000 rpm. Electrospinning will be evenly wound onto the surface of the pol...
Embodiment 3
[0068] Prepare the braided wire for medical use as follows:
[0069] S1. Preparation of electrospinning solution: Polylactic acid was dissolved in hexafluoroisopropanol to make the mass volume concentration of polylactic acid 7%.
[0070] S2. Perform electrospinning. The electrospinning conditions are as follows: the electrospinning voltage is 15 kV, the propulsion speed is 3 mL / h, and the distance from the electrospinning head to the grounded metal plate is 20 cm. After the start of electrospinning, at a distance of about 15 cm from the filament outlet, a bundle of polypropylene threads continued to pass in parallel, and the polypropylene thread was continuously conveyed from one end to the other; at the same time, the polypropylene thread kept rotating itself during the conveying process. The conveying speed of the polypropylene wire was 6 m / min, and the rotation speed of the polypropylene wire was 500 rpm. Electrospinning will be evenly wound onto the surface of the polypr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com