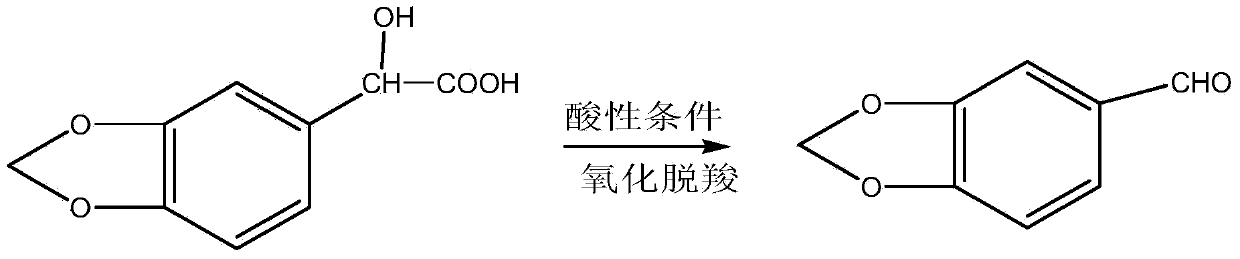
Preparation method and application of sesamol intermediate heliotropin
A technology for piperonal and sesamol is applied in the field of preparation of piperonal, an intermediate product of sesamol, and can solve the problems of large-scale use of high-concentration hydrochloric acid, increase in product impurities, deepening of color, etc., and achieves low raw material cost, simple reaction process, The effect of high decarboxylation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Add 0.98g of concentrated sulfuric acid with a mass fraction of 98% and 2.96g of glyoxylic acid into a self-made reaction vessel equipped with an electric stirrer, and drop into 0.2mL of water, keep the temperature at 0°C, and then Add 4.9g of peppercycline at the speed of drops, mix and stir for 2.5h; the solution at the beginning of the reaction is a colorless and transparent solution, after dropping peppercyclene, it gradually becomes a milky white viscous liquid, and layers, and finally becomes a milky white opaque viscous liquid;
[0027] (2) The above reaction product was diluted with 10mL of water under stirring, then washed by suction filtration, and dried in the air to obtain a white solid powder which was 3,4-methylenedioxymandelic acid;
[0028] (3) Add 19.60 g of 3,4-methylenedioxymandelic acid obtained in step (2) and 5.25 g of nitric acid solution with a mass fraction of 3.1% in a three-neck flask equipped with a stirring and reflux condensing device. ...
Embodiment 2
[0031] (1) Add 0.88g of concentrated sulfuric acid with a mass fraction of 98% and 2.66g of glyoxylic acid into a self-made reaction vessel equipped with an electric stirrer, and drop into 0.2mL of water, keep the temperature at 4°C, and then Add 4.05g of peppercycline at the speed of drops, mix and stir for 3 hours; the solution is a colorless transparent solution at the beginning of the reaction, after dropping peppercyclene, it gradually becomes a milky white viscous liquid, and layers, and finally becomes a milky white opaque viscous liquid;
[0032] (2) The above reaction product was diluted with 10mL of water under stirring, then washed by suction filtration, and dried in the air to obtain a white solid powder which was 3,4-methylenedioxymandelic acid;
[0033] (3) Add 17.64g of 3,4-methylenedioxymandelic acid obtained in step (2) and 5.25g of nitric acid solution with a mass fraction of 3.1% in a three-neck flask equipped with a stirring and reflux condensing device. He...
Embodiment 3
[0036] (1) Add 1.07g of concentrated sulfuric acid with a mass fraction of 98% and 3.25g of glyoxylic acid into a self-made reaction vessel equipped with an electric stirrer, and drop into 0.2mL of water, keep the temperature at 5°C, and then Add 4.95g of peppercycline at the speed of drops, mix and stir for 3.5h; the solution at the beginning of the reaction is a colorless and transparent solution, after dropping peppercyclene, it gradually becomes a milky white viscous liquid, and layers, and finally becomes a milky white opaque viscous liquid;
[0037] (2) The above reaction product was diluted with 10mL of water under stirring, then washed by suction filtration, and dried in the air to obtain a white solid powder which was 3,4-methylenedioxymandelic acid;
[0038] (3) Add 21.56g of 3,4-methylenedioxymandelic acid obtained in step (2) and 5.25g of nitric acid solution with a mass fraction of 3.1% in a three-neck flask equipped with a stirring and reflux condensing device. H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com