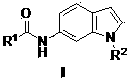
STAT3 (Signal Transducer and Activator of Transcription 3)-targeting small molecular compound as well as preparation method and application thereof
A compound and reaction technology, applied in the field of STAT3 inhibitors, small molecule compounds targeting STAT3 and its preparation, can solve the problems of poor physical and chemical properties, affecting clinical development, low anti-tumor activity of biochemical properties, etc., to achieve the goal of treating tumors effect of disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
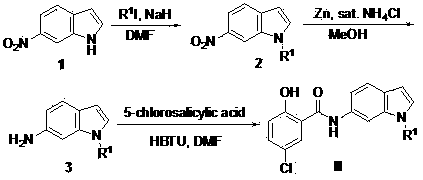
[0032] Example 1: 5-chloro-2-hydroxy- N -(1-Methyl-6-indole)benzamide (preparation of compound IIa)
[0033] Step 1: 1-methyl-6-nitroindole (intermediate 2): 800 mg of 60% NaH was added to 20 mL of DMF solution, and 4 g of 6-nitroindole was added in batches at 0°C. The mixture was stirred at 0°C for 10 minutes, and then 1.86 mL of methyl iodide was added dropwise. After the mixture was stirred at room temperature for 24 hours, it was slowly added to 100 mL of ice water and then 100 mL of ethyl acetate. After the extracted organic phase was washed with saturated sodium chloride, the organic phase was dried with anhydrous sodium sulfate and spin-dried to obtain 4.50 g of intermediate 2. 1 H NMR (400 MHz, CDCl 3 ) δ 8.27 (d, 1H), 7.97 (d, 1H), 7.61 (d, 1H), 7.33 (d, 1H), 6.56 (d, 1H), 3.87 (s, 3H).
[0034] Step 2: 1 g of intermediate 2 was dissolved in 20 mL of methanol, and 100 mg of zinc powder was added. Add 20 mL of saturated ammonium chloride aqueous solution at 0°C,...
Embodiment 2
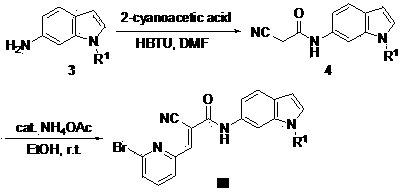
[0036] Example 2: (E)-3-(6-bromopyridine-2)-2-cyano- N -(1-methyl-6-indole)acrylamide (preparation of compound IIIa)
[0037] Step 1 and step 2 are the same as for the synthesis of compound IIa, and the 5-chlorosalicylic acid in step 3 is replaced with 2-cyanoacetic acid. Specifically, 146 mg of intermediate 3 was dissolved in 10 mL of DMF, 100 mg of 2-cyanoacetic acid was added, 500 mg of HBTU and 0.5 mL of DIPEA were added, after stirring at room temperature for 24 hours, 10 mL of water and 30 mL of ethyl acetate were added, and the organic phase obtained by extraction was extracted with 10 mL of water. After the extracted organic phase was washed with saturated sodium chloride, the organic phase was dried with anhydrous sodium sulfate and spin-dried, and the crude product was passed through a silica gel column to obtain 120 mg of intermediate 4. 1 H NMR (400 MHz, DMSO- d 6 ) δ 10.83 (s, 1H), 8.26 (d, 1H), 7.95 (d, 1H), 7.60 (d, 1H), 7.32 (d, 1H), 6.55(d, 1H), 3.97 (s, ...
Embodiment 3
[0039] Example 3: N -(1-methyl-6-indole)-2-phenylquinoline-4-carboxamide (preparation of compound IVa)
[0040] Step 1 and step 2 are the same as the synthesis of compound IIa, and the 5-chlorosalicylic acid in step 3 in the preparation of compound IIa is replaced by 2-phenylquinoline-4-acetic acid. Specifically, 146 mg of intermediate 3 was dissolved in 10 mL of DMF, 300 mg of 2-phenylquinoline-4-acetic acid was added, 500 mg of HBTU and 0.5 mL of DIPEA were added, after stirring at room temperature for 24 hours, 210 mL of water and 50 mL of ethyl acetate were added, and the organic phase obtained by extraction was then Extracted with 20mL of water, the organic phase obtained by the extraction was washed with saturated sodium chloride, dried with anhydrous sodium sulfate and then spin-dried, the crude product was passed through a silica gel column to obtain 220 mg of the final product IVa. 1 H NMR (400 MHz, DMSO- d 6 ) δ 11.31 (s, 1H), 8.45 (s, 1H), 8.39 (d, 2H), 8.35 (s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com