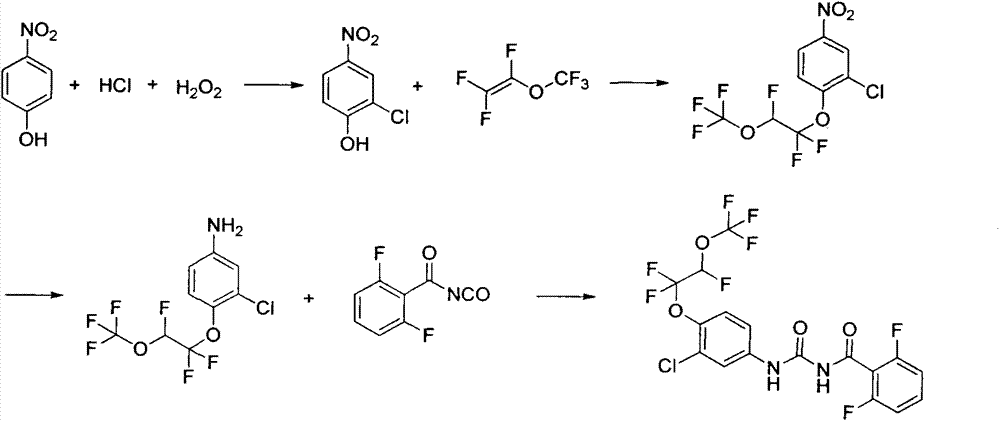
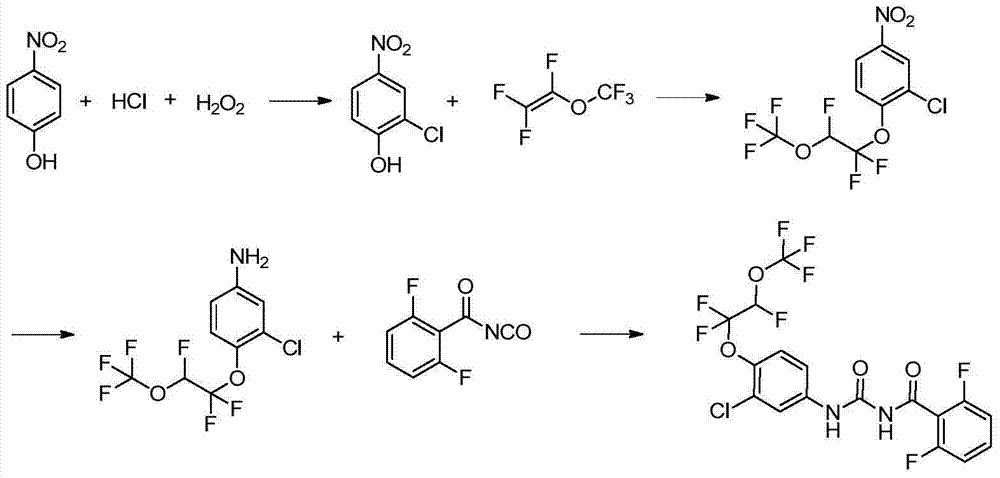
Synthesis method of novaluron
A synthetic method and technology of fluorourea, which is applied in the field of preparation of organic compounds, can solve problems such as side reactions between amino groups and perfluorinated raw materials, increase consumption of perfluorinated raw materials, increase impurity content, etc., so as to avoid side reactions and facilitate industrial production and Environmental protection and the effect of reducing the amount of three wastes produced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Preparation of 2-chloro-4-nitrophenol:
[0027] In a 500ml flask, add 35 grams of p-nitrophenol, 205 grams of concentrated hydrochloric acid, add 26 grams of hydrogen peroxide dropwise under stirring, control the temperature below 30°C, react for 3 hours, filter, wash the filter cake with water until neutral, and dry , recrystallized from dichloroethane to obtain 35 g of yellow solid, yield 80%.
Embodiment 2
[0028] Example 2: Preparation of 2-chloro-4-nitro-1-(1,1,2-trifluoro-2-(trifluoromethoxy)ethoxy)benzene:
[0029] In a 250ml flask, add 3.48g of 2-chloro-4-nitrophenol, 40mL of toluene, 40mL of dimethyl sulfoxide and 0.37g of potassium hydroxide, stir and lower the temperature to 5°C, and introduce 3.32 g of perfluorovinyl methyl ether. g, after aeration, keep warm at 0-10°C for 10 hours, add 50mL of water, separate layers, extract the water phase once with 20mL of toluene, combine the organic phases, dry over anhydrous sodium sulfate, filter, and concentrate to obtain 5.86 g of a yellow-brown solid , yield 86%. 1 HNMR (400MHz, CDCl 3 ): δ5.97(dt, J=56.0, 4.0Hz, 1H), 7.48(d, J=9.2Hz, 1H), 8.13(dd, J=2.8, 9.2Hz, 1H), 8.32(d, J= 2.8Hz, 1H); 19 FNMR δppm -144.84, -86.40, -59.75.
Embodiment 3
[0030] Example 3: Preparation of 2-chloro-4-nitro-1-(1,1,2-trifluoro-2-(trifluoromethoxy)ethoxy)benzene:
[0031]In a 100ml flask, add 6.96g of 2-chloro-4-nitrophenol, 20mL of xylene, 20mL of dimethyl sulfoxide and 1g of potassium hydroxide, stir and lower the temperature to 5°C, and pour in perfluorovinyl methyl ether 6.64 g, after aeration, keep warm at 0-10°C for 10 hours, add 100 mL of water, separate layers, extract the water phase with 50 mL of toluene once, combine the organic phases, dry over anhydrous sodium sulfate, filter, and concentrate to obtain a yellow-brown solid 10.38 grams, yield 76.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com