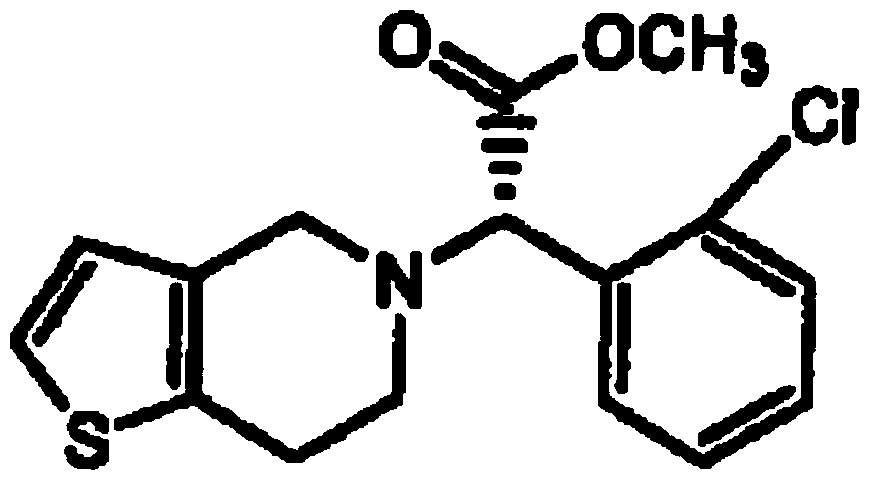
Synthesis method of clopidogrel isomer (+)-(R-)-clopidogrel hydrochloride
A technology of clopidogrel hydrochloride and clopidogrel, which is applied in the field of medicine, can solve the problems of many steps and low product purity, and achieve the effect of mild conditions, simple synthesis steps and high purity of prepared samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
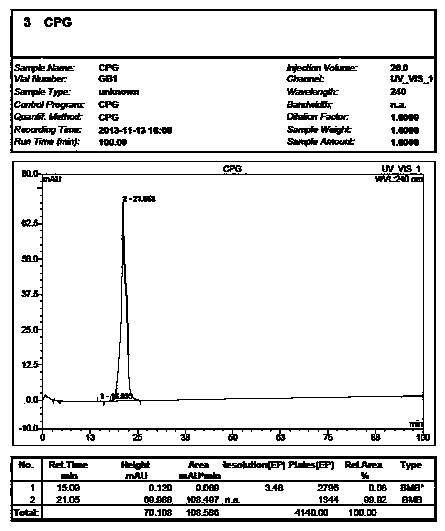
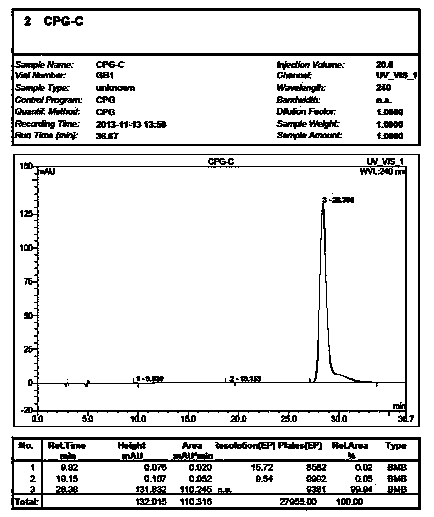
[0015] Weigh 20.0 g of vortex clopidogrel into a 500 ml three-necked flask, add (S+)-camphor-10-sulfonic acid 22 g and acetone 150 ml, heat and reflux at 60 ° C for 3 h, cool down to 0-20 ° C and stir after the reaction is completed After 3 hours, filter, adjust the pH of the filtrate to 7.0-7.2 with aqueous sodium bicarbonate solution, extract with ethyl acetate, and concentrate to obtain an oil.
[0016] Add 20 g of D-di-p-toluoyl tartaric acid and 150 ml of isopropanol to the oil, react at 80°C for 4 hours, cool down to 0-20°C and stir for 3 hours, filter, and dry the crude product.
[0017] Add 150 ml of isopropanol to 20 g of the crude product, and heat to dissolve. Then cool down to 0-10°C for crystallization for 3 hours, and filter. The filter cake was adjusted to pH 7.0-7.2 with aqueous sodium bicarbonate solution, extracted with ethyl acetate, hydrogen chloride gas was passed through, and crystallized at 0-10°C for 3-10 hours. Filter and dry to obtain a white solid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com