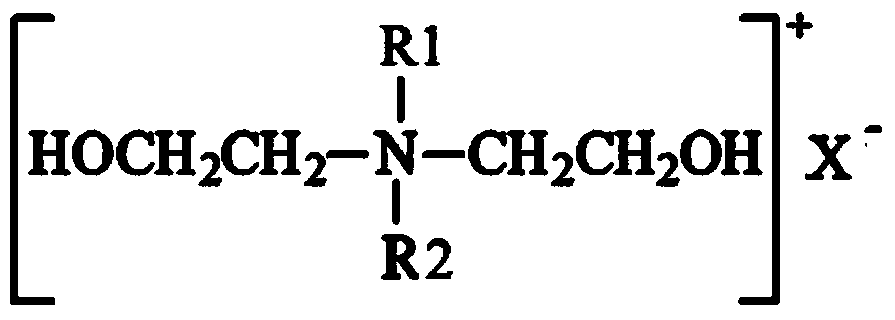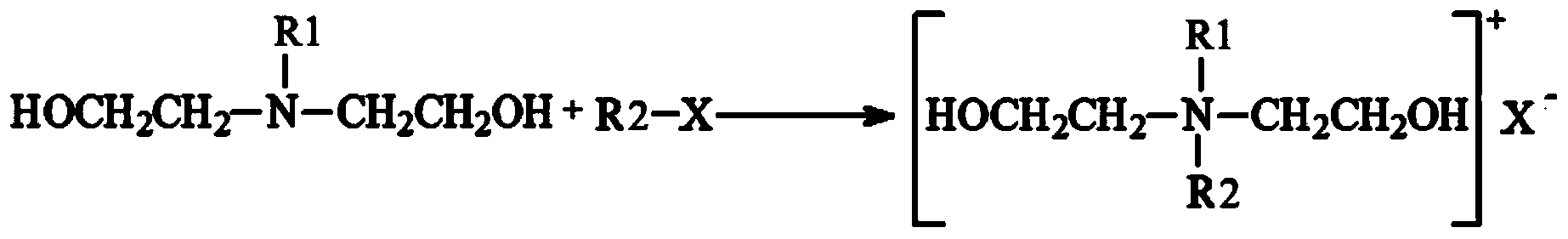Dihydroxyl quaternary ammonium salt with antimicrobial activity as well as preparation method and application thereof
A technology of dihydroxyquaternary ammonium salt and antibacterial activity, which is applied in the field of antibacterial materials and its preparation, bishydroxyquaternary ammonium salt and its preparation, can solve the problems of antibacterial power decline, etc., and achieve fast reaction time, high yield and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
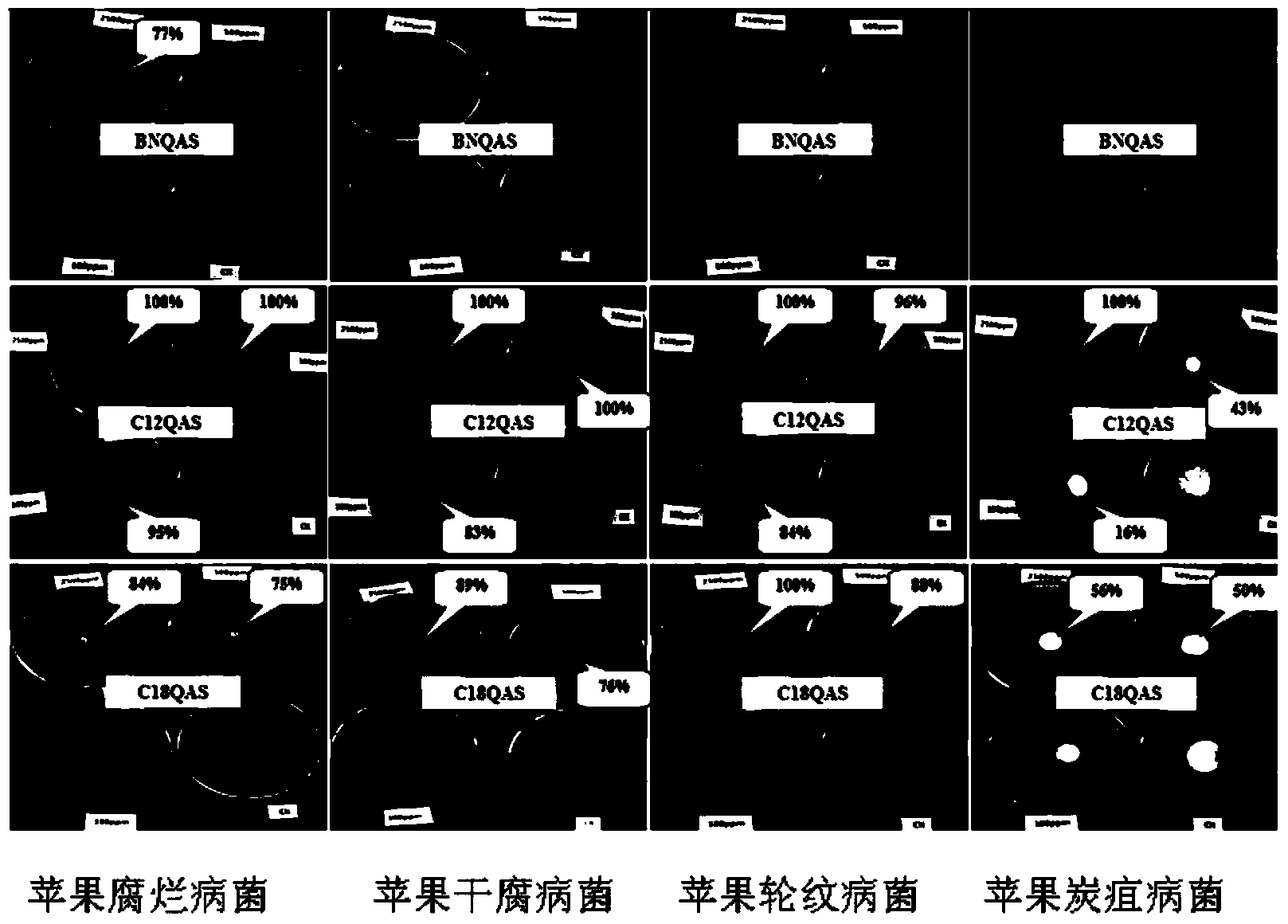
[0026] Embodiment 1: the synthesis of BNQAS
[0027] Dissolve N-methyldiethanolamine (1.19g, 10mmol) in 4mL N,N-dimethylformamide, slowly add benzyl chloride (1.71g, 10mmol) dropwise, and control the reaction temperature at 60°C for 6 hours. Determine the reaction end point by layer analysis (TLC), cool down, add 10ml of anhydrous ether as a precipitant, place in a -20°C refrigerator for 4 hours to obtain a white solid, filter it with suction, and recrystallize the crude product twice with 4ml of absolute ethanol and 30ml of ether 2.7 g of N-methyl-N-benzyl-N,N-dihydroxyethylammonium chloride (BNQAS) was obtained, with a yield of 93%. IR(KBr pellet cm -1 ):3346(br),3045(m),2985(s),2889(m),1618(br),1498(m),1471(s),1384(s),1076(s),906(s ), 760(s), 711(s). 1 H NMR(400MHz,DMSO)δ3.02(s,3H,N-CH 3 ),3.34-3.40(m,2H,N-CH 2 ),3.51-3.56(m, 2H,N-CH 2 ),3.91-3.92(d,4H,2O-CH 2 ),4.71(s,2H,CH 2 ),5.53-5.51(s,2H,2OH),7.51-7.65(m,5H,C 6 h 5 ).Anal.Calcd.for C 12 h 20 NO 2 Br: C49....
Embodiment 2
[0028] Example 2: Synthesis of C12QAS
[0029] Dissolve N-methyldiethanolamine (1.19g, 10mmol) in 4mL N,N-dimethylacetamide, slowly add dodecyl bromide (2.49g, 10mmol) dropwise, and control the reaction temperature at 80°C for 6 hours , determined the end point of the reaction by thin layer analysis (TLC), cooled, added 10ml of anhydrous acetone as a precipitant, and placed it in a -20°C refrigerator for 4 hours to obtain a white solid, filtered it with suction, and weighed the crude product with 5ml of absolute ethanol and 30ml of acetone. Crystallize twice to obtain 3.4 g of N-methyl-N-dodecyl-N,N-dihydroxyethylammonium bromide (C12QAS), with a yield of 92%. IR(KBr pellet cm -1 ):3293(s), 2921(s), 2852(s), 2856(m), 1618(br), 1467(s), 1380(m), 1057(s), 722(s). 1 H NMR (400MHz, DMSO) δ0.84-0.87 (m, 3H, C-CH 3 ),1.25(m,18H,9CH 2 ),1.64-1.71(m,2H,CH 2 ),3.07(s,3H,N-CH 3 ),3.33-3.37(m,2H,N-CH2 ),3.39-3.47(m,4H,2N-CH 2 ),3.81-3.82(m,4H,2O-CH 2 ),5.23-5.26(m,2H,2OH).Anal.Ca...
Embodiment 3
[0030] Example 3: Synthesis of C14QAS
[0031] Dissolve N-acetyldiethanolamine (1.19g, 10mmol) in 5mL of ethanol, slowly add dodecyl bromide (2.77g, 10mmol) dropwise, and control the reaction temperature at 80°C for 8 hours. Through thin layer analysis (TLC ) to determine the end point of the reaction, cool down, add 10ml of anhydrous petroleum ether as a precipitant, place it in a -20°C refrigerator for 4 hours to obtain a white solid, filter it with suction, recrystallize the crude product twice with 5ml of absolute ethanol and 30ml of petroleum ether to obtain N -Acetyl-N-tetradecyl-N,N-dihydroxyethylammonium bromide (C14QAS) product 3.2g, yield 82%. IR(KBr pellet cm -1 ):3289(s), 2920(s), 2847(s), 1618(br), 1467(s), 1377(s), 1047(s), 723(s). 1 H NMR(400MHz,DMSO):δ0.84-0.87(m,3H,C-CH 3 ),1.24-1.27(m,22H,11CH 2 ),1.66-1.67(m,2H,CH 2 ),3.09(s,3H,N-CH 3 ),3.35-3.41(m,2H,N-CH 2 ),3.44-3.45(m,4H,2N-CH 2 ),3.81-3.82(m,4H,2O-CH 2 ),5.23-5.26(m,2H,2OH).Anal.Calcd.for C 19...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com