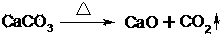Method for extracting soluble potassium sulfate by using orthoclase
A technology of orthoclase and potassium sulfate, applied in the direction of sulfate/bisulfate preparation, etc., can solve the problems of low added value of products, long process flow, poor economic benefits, etc., to achieve increased yield, reasonable process, and improved decomposition rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1, a method for extracting soluble potassium sulfate with orthoclase. After pulverizing and mixing orthoclase, limestone, phosphogypsum, and roasting aids, the soluble potassium sulfate is obtained through high-temperature roasting, crushing, leaching, and evaporation processes. Potassium sulfate product crystallization;
[0024] The weight ratio of limestone, orthoclase and phosphogypsum is 0.5:1:0.2;
[0025] Roasting aid weight is 0.1% of orthoclase;
[0026] The roasting aid is selected from one of potassium sulfate, sodium sulfate, and fluorite;
[0027] The temperature of the high-temperature calcination is 500°C.
Embodiment 2
[0028] Embodiment 2, a method for extracting soluble potassium sulfate with orthoclase, after pulverizing and mixing orthoclase, limestone, phosphogypsum, and roasting aids, then undergoing high-temperature roasting, crushing, leaching, and evaporation processes to obtain soluble potassium sulfate Potassium sulfate product crystallization;
[0029] The weight ratio of limestone, orthoclase and phosphogypsum is 4.9:1:2.5;
[0030] Roasting aid weight is 20% of orthoclase;
[0031] The roasting aid is selected from one of potassium sulfate, sodium sulfate, and fluorite;
[0032] The temperature of high temperature calcination is 1200°C.
Embodiment 3
[0033] Example 3, a method for extracting soluble potassium sulfate with orthoclase. After pulverizing and mixing orthoclase, limestone, phosphogypsum, and roasting aids, the soluble potassium sulfate is obtained through high-temperature roasting, crushing, leaching, and evaporation processes. Potassium sulfate product crystallization;
[0034] The weight ratio of limestone, orthoclase and phosphogypsum is 1.5:1:1.0;
[0035] Roasting aid weight is 5% of orthoclase;
[0036] The roasting aid is selected from one of potassium sulfate, sodium sulfate, and fluorite;
[0037] The temperature of the high-temperature calcination is 850°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com