Method of producing hydrogen chloride
A manufacturing method, hydrogen chloride technology, applied in hydrogen chloride preparation, chlorine/hydrogen chloride, chloride preparation, etc., can solve problems such as poor energy efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
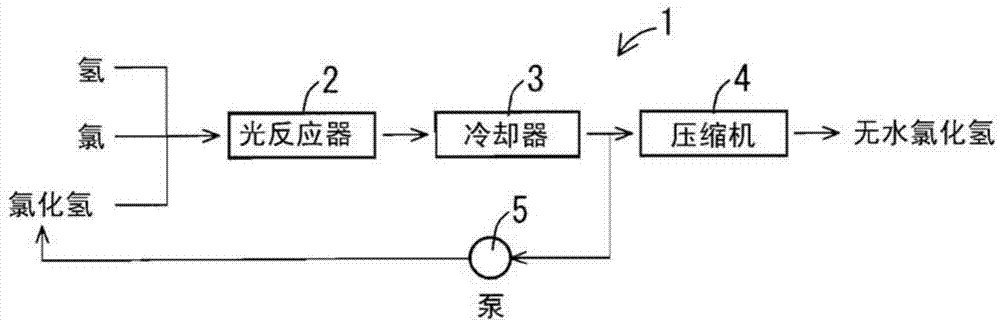
[0066] A cylindrical flask made of heat-resistant glass with a diameter of 20 cm and a height of 50 cm provided with two inlets for raw material gas at the upper part and one outlet for the reaction product at the lower part was used as the photoreactor 2 . The raw material gas is blown into the photoreactor 2 so that the raw material gas introduced from the raw material gas inlet port swirls in the circumferential direction on the upper part of the photoreactor 2 . The raw material gases were continuously supplied from the gas cylinders in the following supply amounts. The raw material hydrogen was supplied from one inlet at a flow rate of 6 NL / min. A mixed gas of chlorine gas and hydrogen chloride gas was supplied from another inlet at a flow rate of 5 NL / min for chlorine gas and 120 NL / min for hydrogen chloride gas. The unit "NL / min" is a flow unit expressed by the volume (liter) that the gas in the standard state flows per minute.
[0067] In the center of the photoreact...
Embodiment 2)
[0069] Instead of arranging a high-pressure mercury lamp as a light source in the photoreactor 2 , it was arranged outside the photoreactor 2 and light was irradiated from the outside, and the procedure was carried out in the same manner as in Example 1. A 100W high-pressure mercury lamp (M-102, manufactured by Ushio Electric Co., Ltd.) was arranged at a distance of 1 m from the center of the photoreactor 2 . The temperature of the raw material gas was 30°C, and the temperature of the mixture containing hydrogen chloride gas derived from the photoreactor 2 after the reaction was 190°C. The composition of the mixture derived from the photoreactor 2 was 0.7 vol % of hydrogen, 99.3 vol % of hydrogen chloride, and 50 ppm of chlorine.
Embodiment 3)
[0071]The same procedure as in Example 2 was carried out except that the flow rate of introduced hydrogen gas was 16.5 NL / min, the flow rate of chlorine gas was 15 NL / min, and the flow rate of hydrogen chloride gas was 120 NL / min. The temperature of the raw material gas was 30°C, and the temperature of the mixture containing hydrogen chloride gas derived from the photoreactor 2 after the reaction was 480°C. The composition of the mixture derived from the photoreactor 2 was 1.0 vol % of hydrogen, 99.0 vol % of hydrogen chloride, and no chlorine was observed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com