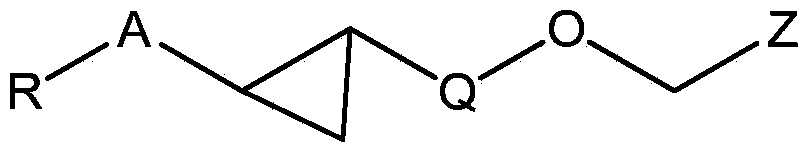
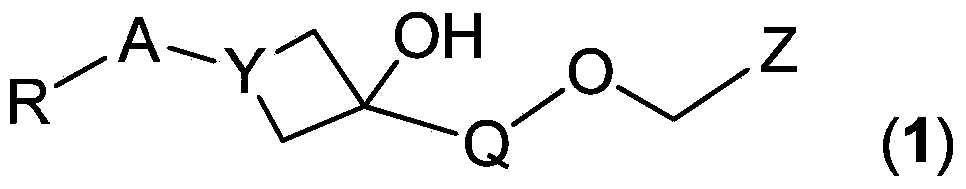
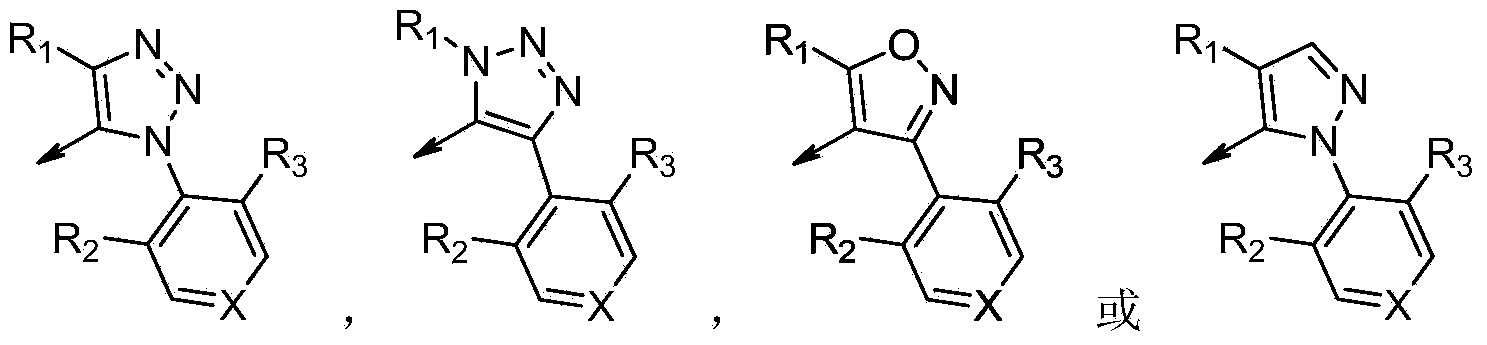
Novel fxr (nr1h4) binding and activity modulating compounds
A technology of compounds and solvates, applied in the field of compounds of agonists or modulators, which can solve problems such as low levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Example 1: 3-((1s , 3s)-3-(2-chloro-4-((5-cyclopropyl-3-(2 , 6-dichlorophenyl) isoxazol-4-yl) methoxy) phenyl) -3- Methyl hydroxycyclobutyl)benzoate (1)
[0130]
[0131] Step 1: 4-((4-Bromo-3-chlorophenoxy)methyl)-5-cyclopropyl-3-(2,6-dichlorophenyl)-isoxazole (1a)
[0132] To (5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl)methanol (13g, 45.8mmol) in CH 2 Cl 2 SOCl was added dropwise to a solution in (DCM) (200 mL) 2 (40 mL, 336 mmol). The resulting mixture was stirred at room temperature for 24 hours, and the solvent was removed under reduced pressure. The residue was dissolved in N,N-dimethylformamide (DMF) (200 mL), and to this solution was added 4-bromo-3-chlorophenol (9.7 g, 47 mmol), K 2 CO 3(40g, 290mmol) and NaI (12g, 80mmol). The mixture was stirred at 60 °C overnight, then cooled to room temperature, diluted with water (1000 mL) and extracted with ethyl acetate (EA) (500 mL×3). The organic phases were combined, washed with brine (500 mL...
Embodiment 2
[0139] Example 2: 3-((1s,3s)-3-(2-chloro-4-((5-cyclopropyl-3-(2,6-dichlorophenyl)isoxazol-4-yl) Methoxy)phenyl)-3- Hydroxycyclobutyl)benzoic acid (2)
[0140]
[0141] Compound 1 (1.7 g, 2.84 mmol) was dissolved in THF (100 mL) at room temperature. A solution of LiOH (285 mg, 4.2 equiv) in water (20 mL) was added and the solution was stirred and warmed to 35 °C for three days. After this time, THF was removed under reduced pressure. The remaining aqueous solution was diluted with water (25 mL) and washed with Et 2 O (2x50 mL) washes. The aqueous layer was then transferred to a round bottom flask and acidified to pH 6 using 1N HCl. The formed white precipitate was filtered off, and dried under reduced pressure at 50° C. to obtain the title compound 2 (1.3 g, 78%, passed 1 Single isomer by H-NMR and LC-MS) as a white solid. 1H NMR (400MHz, CD3OD) δ: 7.98 (s, 1H), 7.86 (d, J=7.6Hz, 1H), 7.58-7.46 (m, 5H), 7.41 (t, J=7.6Hz, 1H), 6.91 (d, J=2.4Hz, 1H), 6.80 (dd, J=8.8...
preparation Embodiment 3
[0154]
[0155] Step 1: Methyl 3-(3-hydroxyazetidin-1-yl)benzoate (3a)
[0156] To a solution of methyl 3-iodobenzoate (4.5 g, 17.2 mmol) in DMSO (30 mL) was added 3-azetidin-3-ol hydrochloride (1.3 g, 11.8 mmol), Cs 2 CO 3 (9.5g, 29.2mmol), CuI (446mg, 2.3mmol) and L-proline (540mg, 4.7mmol), and the mixture was heated at 90°C for 18 hours under an argon atmosphere. The solution was diluted with EA and water, then the organic layer was washed three times with brine, concentrated under reduced pressure, and purified with CC (PE / EA=2:1) to give compound 3a (1.6 g, 66%) as a yellow solid.
[0157] Step 2: Methyl 3-(3-oxoazetidin-1-yl)benzoate (3)
[0158]To a solution of compound 3a (1.60 g, 7.7 mmol) in dry DCM (30 mL) was added Dess-Martin periodinane (6.5 g, 15.4 mmol) at 0 °C and incubated under N 2 The mixture was stirred at room temperature under atmosphere for 2 hours. The mixture was quenched with saturated sodium bicarbonate solution and diluted with EA. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com