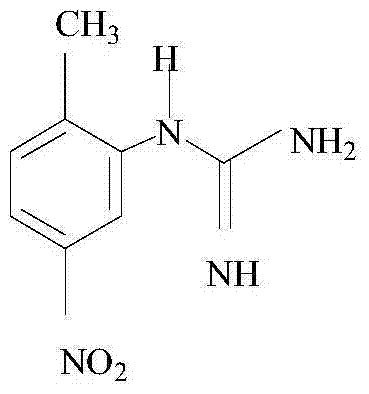
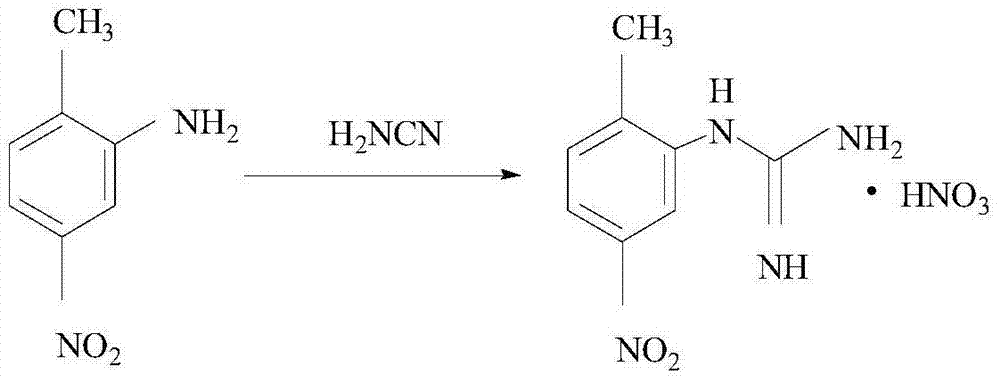
Synthetic method of (2-methyl-5-nitro phenyl) guanidine sulfate
A technology of nitrophenyl and synthesis method, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of low product purity, low yield, long reaction time, etc., and achieve high product purity, The effect of high yield and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 20g (0.131mol) of 2-amino-4 nitrotoluene into a three-necked flask, add 30ml of ethanol and 8ml of DMF, adjust the base to pH=12 with 10% NaOH, stir for 20min, add 33g (0.134mol) of oxymethylisourea Sulphate, heated to reflux, reacted for 5h; after the reaction, use 8%H 2 SO 4 Adjust the acid to pH = 6.5, and then keep it warm for 40 minutes; after the keep warm, cool down to 0°C, filter with suction, wash twice with 120ml ethanol, and dry to get 31.16g of solid product with a purity of 99.7% and a yield of 83.0%.
Embodiment 2
[0021] Add 20g (0.131mol) of 2-amino-4 nitrotoluene into a three-necked flask, add 35ml of ethanol and 6ml of DMF, adjust the base to pH=11 with 25% ammonia water, stir for 30min, add 32g (0.131mol) of oxymethylisourea Sulphate, heated to reflux, reacted for 6h; after the reaction, use 10%H 2 SO 4 Adjust the acid to pH=7.0, and then keep it warm for 30 minutes; after the keep warm, cool down to 0°C, filter with suction, wash twice with 100ml ethanol, and dry to get 31.85g of solid product with a purity of 99.8% and a yield of 81.2%.
Embodiment 3
[0023] Add 20g (0.131mol) of 2-amino-4 nitrotoluene into a three-necked flask, add 38ml of ethanol and 5ml of DMF, adjust the base to pH=13 with 25% ammonia water, stir for 30min, add 35g (0.144mol) of oxymethylisourea Sulphate, heated to reflux, reacted for 8h; after the reaction, use 10%H 2 SO 4 Adjust the acid to pH=6.0, and then keep it warm for 30 minutes; after the keep warm, cool down to 0°C, filter with suction, wash twice with 100ml ethanol, and dry to get 32.62g of solid product with a purity of 99.9% and a yield of 85.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com