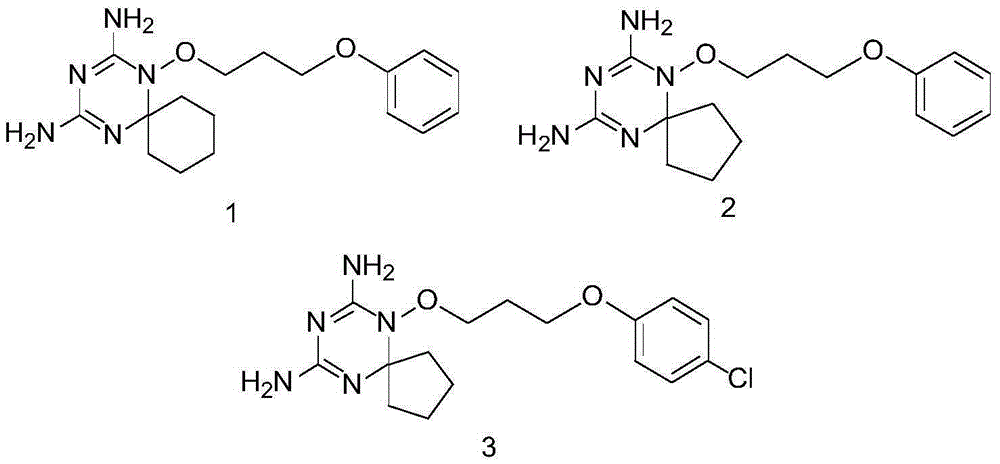
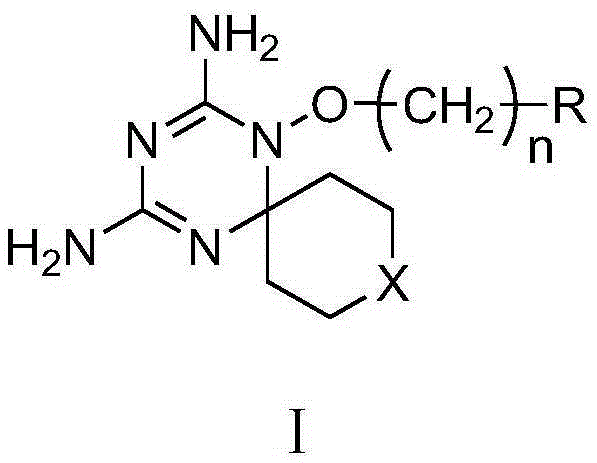
Diaminodihydrotriazine derivative, its salt, preparation method, composition and application
A technology of diaminodihydrotriazine and derivatives, which is used in drug combinations, active ingredients of heterocyclic compounds, bone diseases, etc., can solve the problem of difficult to find out the effect of activity, and has not been studied related to triazine spiroheterocyclic compounds. Properties, no reports on the structure-activity relationship of diaminodihydrotriazine spiroheterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] 1-Bromo-3-phenoxypropane
[0176] Phenol (4.7g, 0.05mol), 1,3-dibromopropane (50g, 0.25mol), and potassium carbonate (10g, 0.075mol) were mixed in 100ml of acetonitrile, and reacted at 80°C for 9 hours. When the phenol is consumed as monitored by TLC, stop the reaction, place it at room temperature, remove the solid by suction filtration, concentrate the filtrate under reduced pressure, add 100ml of chloroform, wash the chloroform layer twice with 0.2N aqueous sodium hydroxide solution (100ml*2), wash with anhydrous magnesium sulfate to dry. Distilled under reduced pressure, under the pressure of 0.48mbar, the fraction at 76-78°C was taken to obtain 6.7g of colorless oil, with a yield of 62.2%. References for specific methods (Hideki Kubota, Toshihiro Watanabe. Synthesis and pharmacological evaluation of N-acyl-1,2,3,4-tetrahydroisoquinoline derivatives as novel specific bradycardicagents. Bioorganic & Medicinal Chemistry, 2004, 12:871-882).
[0177] Compounds of gene...
Embodiment 2
[0179] 1-bromo-3-(2’,4’-dichlorophenoxy)propane
[0180] 2,4-dichlorophenol (8.2g, 0.05mol), 1,3-dibromopropane (50g, 0.25mol), and potassium carbonate (10g, 0.075mol) were mixed in 100ml of acetonitrile and reacted at 80°C for 8 hours. When the phenol is consumed as monitored by TLC, stop the reaction, place it at room temperature, remove the solid by suction filtration, concentrate the filtrate under reduced pressure, add 100ml of chloroform, wash the chloroform layer twice with 0.2N aqueous sodium hydroxide solution (100ml*2), wash with anhydrous magnesium sulfate to dry. Distilled under reduced pressure, under 0.3mbar pressure, the fraction at 106-108°C was taken to obtain 8.5g of colorless oily substance, with a yield of 60.5%.
Embodiment 3
[0182] 1-Bromo-3-(4’-methoxyphenoxy)propane
[0183] 4-Methoxyphenol (6.2g, 0.05mol), 1,3-dibromopropane (50g, 0.25mol), and potassium carbonate (10g, 0.075mol) were mixed in 100ml of acetonitrile, and reacted at 80°C for 9 hours. When the phenol is consumed as monitored by TLC, stop the reaction, place it at room temperature, remove the solid by suction filtration, concentrate the filtrate under reduced pressure, add 100ml of chloroform, wash the chloroform layer twice with 0.2N aqueous sodium hydroxide solution (100ml*2), wash with anhydrous magnesium sulfate to dry. Distilled under reduced pressure, under 0.5mbar pressure, the fraction at 98-102°C was taken to obtain 8.1g of colorless oily substance, with a yield of 66.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com