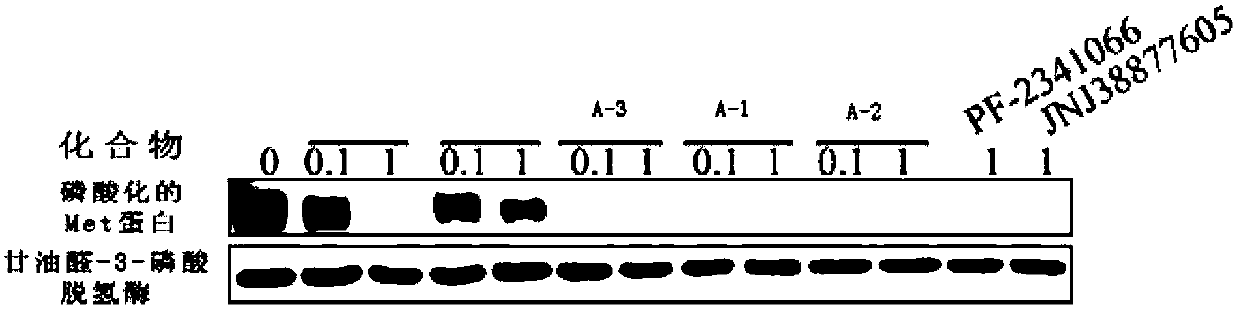
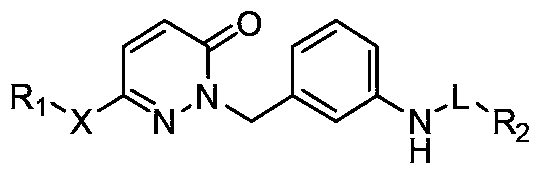
Pyridazinone compounds, their preparation methods, pharmaceutical compositions and uses thereof
The technology of a compound, pyridazinone, applied in the field of medicinal chemistry, can solve the problems of large difference in activity level at the cell level and poor physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0124] Preparation Example 1: Preparation of Intermediate I-1:
[0125]
[0126] Put 40ml of DMF in a round bottom flask, slowly add 6.89ml of POCl under ice-cooling 3 (Phosphorus oxychloride), after the dropwise addition, after stirring in an ice bath for 35min, slowly add a DMF solution of 5g of 6-fluoroindole in a constant pressure dropping funnel, and stir for 40min at 35°C. The reaction solution gradually changed from colorless to red. After TLC showed that the reaction was complete, 100 ml of 19.3 mmol / L NaOH aqueous solution was added, and vigorously stirred at 80° C. for 30 min. The reaction solution was cooled, and ethyl acetate was added for extraction, 30 ml each time, for a total of three extractions. The organic layers were combined, dried over anhydrous sodium sulfate, evaporated to dryness, and subjected to silica gel column chromatography to obtain 4.5 g of intermediate I-1 (white solid, yield 75%).
[0127] 1 H NMR (300MHz,D 2 O)δ10.01(s,1H),8.24(dd,J=...
preparation Embodiment 2
[0128] Preparation Example 2: Preparation of Intermediate I-2:
[0129]
[0130]Dissolve 1.5g of 3-formyl-6-fluoroindole (ie intermediate I-1) in isopropanol, add 150mg of palladium carbon and 3.2g of sodium borohydride, and reflux overnight at 80°C. After TLC showed that the substrate disappeared, the palladium carbon was removed by filtration. After the filtrate was concentrated, the excess sodium borohydride was quenched by adding water, and extracted with ethyl acetate, 15 ml each time, three times. The organic layers were combined, dried over anhydrous sodium sulfate, evaporated to dryness, and silica gel column chromatography to obtain intermediate I-2 (white solid, quantitative yield).
[0131] 1 H NMR (300MHz, CDCl 3 )δ7.60(s,1H),7.56(dd,J=7.5,5.1Hz,1H),7.03(dd,J=8.0,1.4Hz,1H),6.98–6.90(m,2H),2.34(s ,3H).
preparation Embodiment 3
[0132] Preparation Example 3: Preparation of Intermediate I-3:
[0133]
[0134] 1 g of 3-methyl-6-fluoroindole (ie intermediate I-2) and 1 g of dichloropyridazine were dissolved in reevaporated and dried THF under nitrogen protection. Add 270 mg NaH under ice cooling and stir rapidly. After the reaction was stable, the ice bath was removed and stirred at room temperature. After the complete conversion of the substrate was detected by TLC, excess water was added to quench the unreacted NaH, and extracted with ethyl acetate, 20 ml each time, twice. The combined organic layers were dried and concentrated. The crude product was dissolved in glacial acetic acid and refluxed at 120 °C overnight. After stopping the reaction, the glacial acetic acid was distilled off under reduced pressure, and the crude product was dissolved in 100ml of ethyl acetate, washed 5 times with 50ml of water each time. The organic layer was dried, concentrated, and subjected to silica gel column chr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com