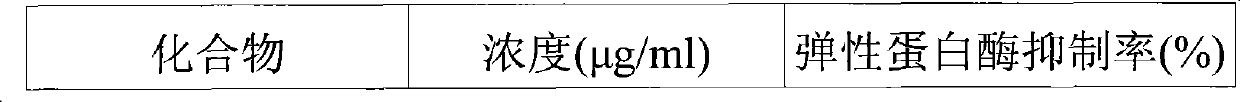New application of phytosterol functioning as elastinase activity inhibitor, and composition containing the same
A kind of technology of elastase and activity inhibitor, applied in the field of phytosterol and its composition as an active ingredient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
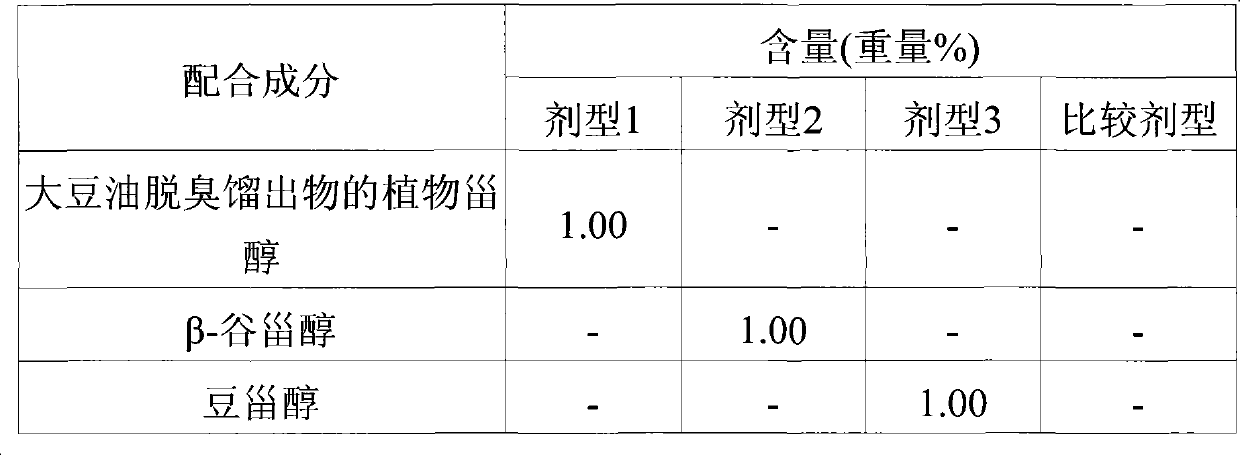
[0025] [Example 1] Production of phytosterols from soybean oil deodorized distillates
[0026] Pour 200g of soybean oil deodorized distillate (phytosterol content: 27%) and 200g of toluene into a 3L three-necked flask, stir at 300rpm and heat up to 90°C, then add 200g of 25% (w / w) NaOH solution. Thereafter, the reaction product was stirred at 300 rpm at 80° C., 150 g of a 50% (w / w) sulfuric acid solution was slowly added, stirred for 15 minutes, and left to stand to remove the water layer to obtain an oil layer. The oil layer was washed repeatedly until the sulfuric acid was completely removed, and then filtered under reduced pressure at 110°C to remove toluene. Mix 300g of methanol on the above filtrate, pour it into a 3L three-neck flask, react at 65°C for 30 minutes, cool to room temperature, and stir for 24 hours, filter the fixed product generated during the stirring process, and wash it with 200g of methanol , the solid product is distilled under reduced pressure to com...
Embodiment 2
[0027] [Example 2] Isolation and Confirmation of Phytosterols
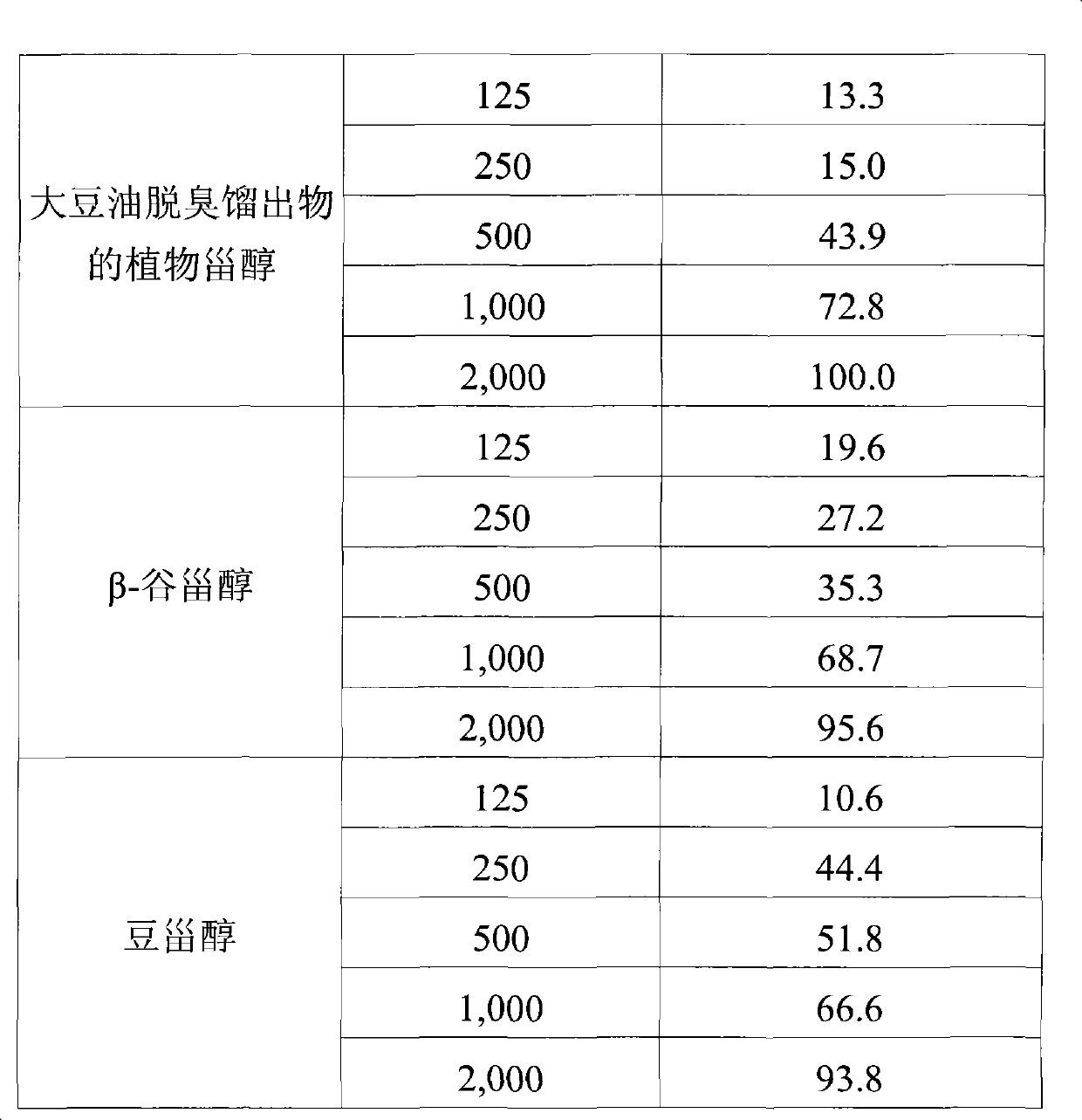
[0028] 1.5 g of the above-mentioned phytosterols were separated by C18 column chromatography. At this time, the used column eluate starts with a solvent in which methanol and water are each mixed in a volume ratio of 80:20 (v / v), and gradually increases the ratio of methanol within 40 minutes so that methanol becomes 100%. The speed and detection wavelength are 60ml / min and UV 210nm respectively. After the above C18 column chromatography separation, 0.75 g of β-sitosterol and 0.38 g of stigmasterol were obtained from subfractions 48-52 and 55-60.
[0029] The instrumental analysis results of the separated phytosterols are as follows.
[0030] Compound 1 (β-sitosterol)
[0031] 1 H-NMR (CDCl 3 , 600MHz) δ3.53(tdd, 1H, J=4.5, 4.2, 3.8Hz, H-3), δ5.36(t, 1H, J=6.4Hz, H-5), δ0.93(d, 3H , J=6.5Hz, H-19), δ0.84(t, 3H, J=7.2Hz, H-24), δ0.83(d, 3H, J=6.4Hz, H-26), δ0.81 (d, 3H, J=6.4Hz, H-27), δ0.68(s, 3H, H-28), δ1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com