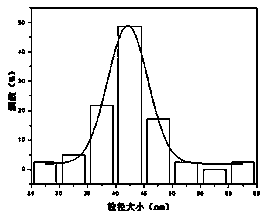
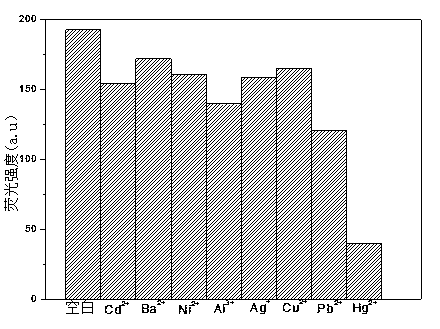
Method for detecting Hg<2+> by fluorescence quenching
A fluorescence quenching and detection method technology, applied in the field of physical chemistry, can solve the problems of high cost and complicated operation, and achieve the effects of low cost, high sensitivity and selectivity, and short time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Detection of Yellow River Raw Water Samples in Qilihe District, Lanzhou Section
[0027] After the Yellow River water samples were collected, they were filtered three times with absorbent cotton to remove a large amount of sediment, and stored in 4 0 C refrigerator is used as a test water sample for backup, prepare a number of semiconductor quantum dots containing about 1.8mg / mL, add 1~3mL PBS buffer solution (pH=7.04,) to it and shake well, measure the emission spectrum at a certain excitation wavelength, and then gradually Accumulatively add 8~12uL of the original water sample of the Yellow River, and the change results of the fluorescence intensity at the same excitation wavelength after adding different volumes of the Yellow River water sample to a certain molar amount of semiconductor quantum dots are shown in Figure 5 . Figure 5 In the same concentration of CdSe-CdS-NAC solution, after adding different volumes of Yellow River water samples, the change diagram ...
Embodiment 2
[0029] Detection of Raw Water Samples of the Yellow River in Chengguan District, Lanzhou Section
[0030] After the Yellow River water samples were collected, they were filtered three times with absorbent cotton to remove a large amount of sediment, and stored in 4 0 C refrigerator is used as a test water sample for backup, prepare a number of semiconductor quantum dots containing about 1.8mg / mL, add 1~3mL PBS buffer solution (pH=7.04,) to it and shake well, measure the emission spectrum at a certain excitation wavelength, and then gradually Accumulatively add 8~12uL of raw water samples from the Yellow River, and measure the change of fluorescence intensity at the same excitation wavelength after adding a certain molar amount of semiconductor quantum dots to different volumes of Yellow River water samples. By calculating the Hg in the water samples of the Yellow River 2+ The content is about 3.10~9.85ppm.
Embodiment 3
[0032] Detection of raw water samples of the Yellow River in Duanyantan, Lanzhou
[0033] After the Yellow River water samples were collected, they were filtered three times with absorbent cotton to remove a large amount of sediment, and stored in 4 0 C refrigerator is used as a test water sample for backup, prepare a number of semiconductor quantum dots containing about 1.8mg / mL, add 1~3mL PBS buffer solution (pH=7.04,) to it and shake well, measure the emission spectrum at a certain excitation wavelength, and then gradually Cumulatively add 8~12uL of raw water samples from the Yellow River, and measure the change in fluorescence intensity at the same excitation wavelength after adding a certain molar amount of semiconductor quantum dots to different volumes of Yellow River water samples. By calculating the Hg in the water samples of the Yellow River 2+ The content is about 3.12~9.88ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com