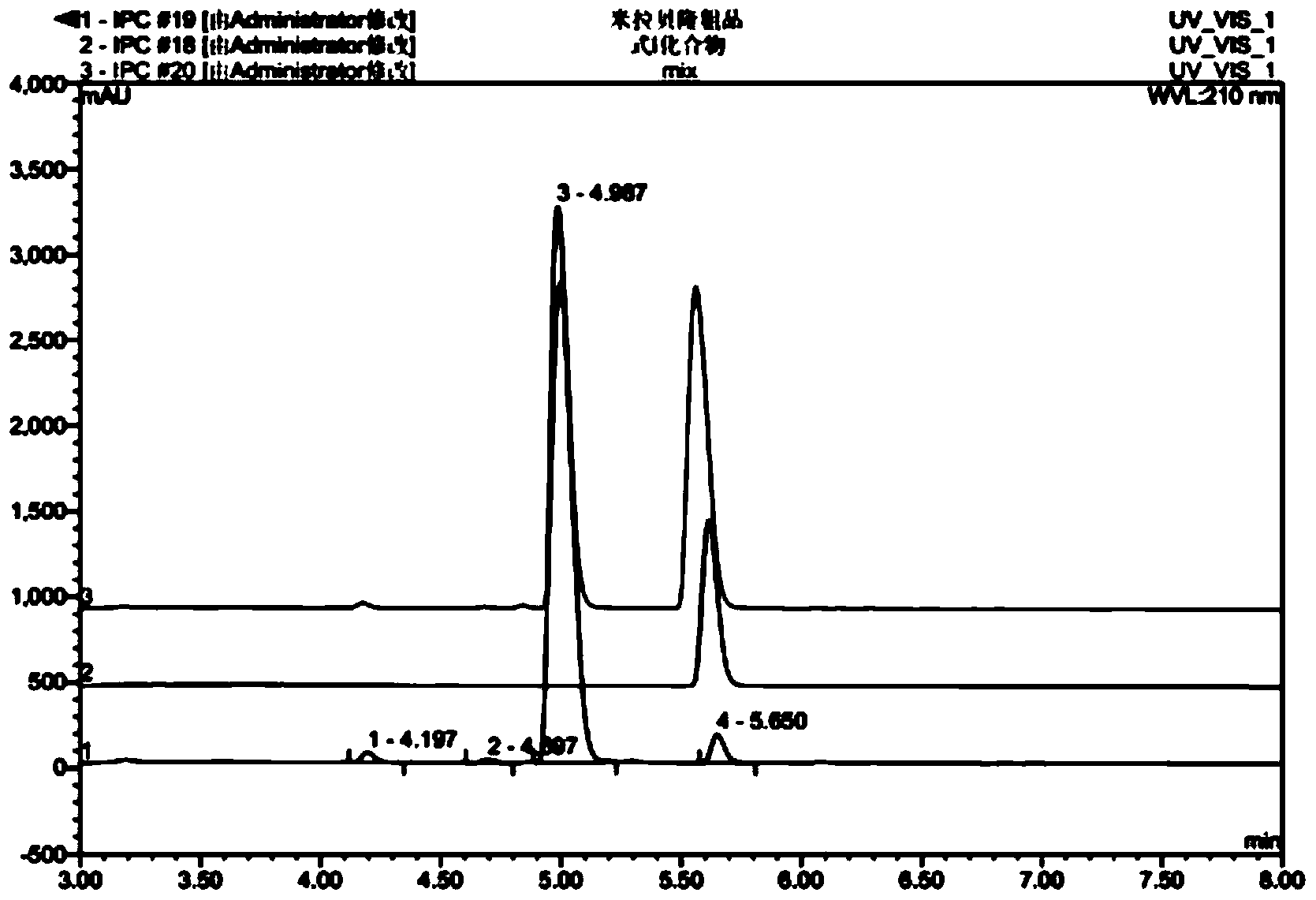
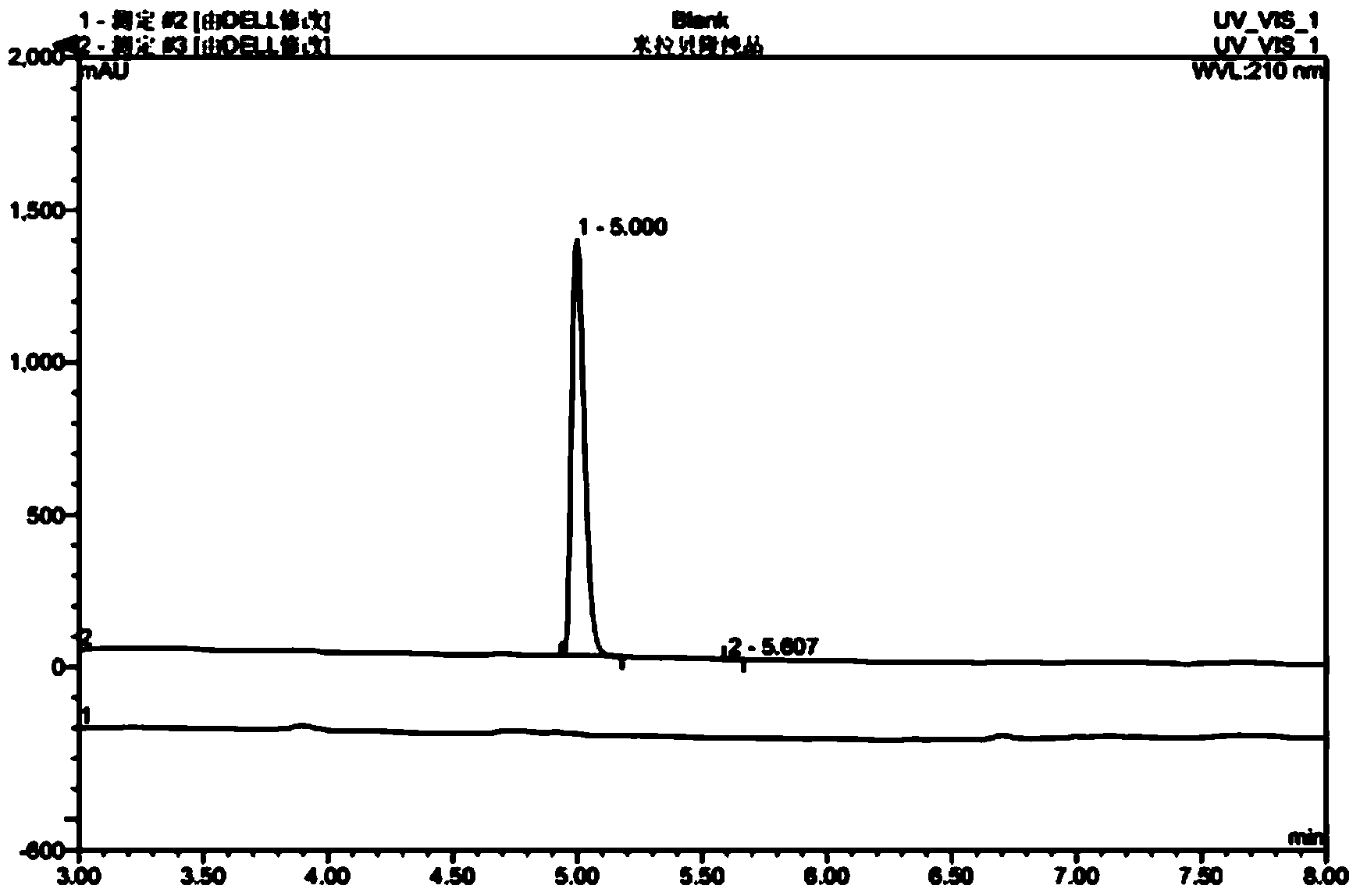
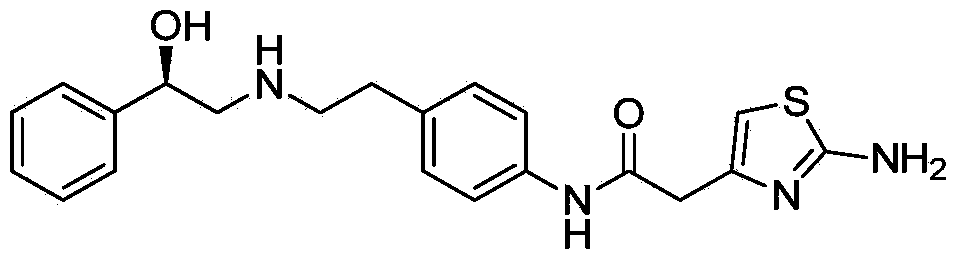
Mirabegron related substance or salt thereof, and preparation method and use thereof
A related substance, mirabegron technology, applied in carboxylate preparation, organic chemistry, etc., can solve problems such as ineffective identification and quality control, and generation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The preparation of embodiment 1N-(4-nitrophenylethyl)-2-phenylacetamide (compound shown in formula 4)
[0056] The compound shown in formula 2 (10.0g, 0.073mol) and the compound shown in formula 3 (14.88g, 0.073mol) were added to DMF (50mL), followed by triethylamine (7.43g, 0.073mol) , 1-Hydroxybenzotriazole (9.93g, 0.073mol), 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (14.78g, 0.077mol) Added to the above reaction solution, stirred at room temperature for 18h. Add water and dichloromethane to extract. The organic layer was dried, filtered, and concentrated under reduced pressure. Recrystallization from toluene gave a white solid (17.33g, 82.99%).
[0057] mp130.2~131.0℃.
[0058] ESI-MS(m / z):285[M+H] + ,307[M+Na] + .
[0059] 1 H NMR (400MHz, DMSO-d 6 )δ:2.84~2.88(t,2H,NO 2 PhCH 2 ),3.29(s,2H,COCH 2 ), 3.34~3.39(m, 2H, NCH2), 7.19~7.28(m, 5H, Ph-H), 7.42~7.44(d, 2H, nitrobenzene meta H), 8.04(s, 1H, heavy water exchange Signal disap...
Embodiment 2
[0060] The preparation of embodiment 2N-(4-nitrophenylethyl)-2-phenethylamine hydrochloride (the hydrochloride of the compound shown in formula 5)
[0061] Add the compound shown in Formula 4 (5.0g, 17.59mmol), anhydrous THF (15mL), 1,3-dimethyl-2-imidazolidinone (15ml) into a three-necked flask, protect it with nitrogen, and cool to Add 1M BH dropwise after -20℃ 3 -THF solution (38.69mL, 38.69mmol), after dropping, stirred at 65°C for 5h. The reaction solution was cooled to -15°C, and methanol (2.5 mL) and concentrated hydrochloric acid (3.3 mL) were added dropwise. After dropping, stir at 65°C for 1 h, concentrate under reduced pressure, add 20% potassium carbonate solution (50 mL) to the residue, extract with ethyl acetate (3×25 mL), wash the organic layer with saturated brine, dry, filter, and concentrate under reduced pressure . The residue was dissolved in isopropanol (50 mL), crystallized with concentrated hydrochloric acid (1.45 mL), filtered, the filter cake was wa...
Embodiment 34
[0064] Preparation of Example 34-[2-(phenylethylamino)ethyl]aniline hydrochloride (the hydrochloride of the compound shown in formula 6)
[0065] Add the compound shown in Formula 5 (3.5g, 11.41mmol), 10%Pd / C (0.35g) into methanol, pass in hydrogen, stir for 12h, filter the reaction solution, concentrate the filtrate under reduced pressure, and wash the residue with methanol (10mL ) was dissolved, crystallized by adding isopropyl ether (70mL) dropwise, filtered, the filter cake was washed with isopropyl ether, and dried in vacuo to obtain a white solid (2.9g, 91.83%).
[0066] mp186°C (decomposition). ESI-MS(m / z):241[M+H] + .
[0067] 1 H NMR (400MHz, DMSO-d 6 )δ:2.80~3.16(m,8H,4CH 2 ),4.99(br s,2H,NH 2), 6.53~6.55(d, 2H, aniline ortho-H), 6.90~6.92(d, 2H, aniline meta-position H), 7.24~7.36(m, 5H, Ph-H), 9.22(br s, 2H, NH 2 + Cl - ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com