Actinic-ray- or radiation-sensitive resin composition, actinic-ray- or radiation-sensitive film therefrom and method of forming pattern
A resin composition, actinic ray technology, applied in the direction of photosensitive materials for optomechanical equipment, optomechanical equipment, originals for optomechanical processing, etc., can solve problems such as resolution drop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example
[0363] Specific examples thereof include 2-[2-{2-(2,2-dimethoxy-phenoxyethoxy)ethyl}-bis-(2-methoxyethyl)]-amine and Compounds (C1-1) to (C3-3) given by way of examples in paragraph [0066] of Patent Application No. 2007 / 0224539A1.
[0364] (4) Ammonium salt
[0365] Ammonium salts may also be suitably used. Ammonium hydroxide and ammonium carboxylates are preferred. A particularly preferred example thereof is tetraalkylammonium hydroxide, such as tetrabutylammonium hydroxide.
[0366] As other basic compounds usable in the composition of the present invention, compounds synthesized in Examples of JP-A-2002-363146, described in paragraph [0108] of JP-A-2007-298569 compounds, etc.
[0367] In addition, a photosensitive basic compound can be used as the basic compound. As the photosensitive basic compound, there can be used, for example, compounds described in Japanese PCT National Publication No. 2003-524799, J. Photopolym. Sci & Tech. Vol. 8, pp. 543-553 (1995) and the lik...
Embodiment
[0599] The invention will be described in more detail below by way of its examples. However, the gist of the present invention is not limited to these Examples in any way.
[0600]
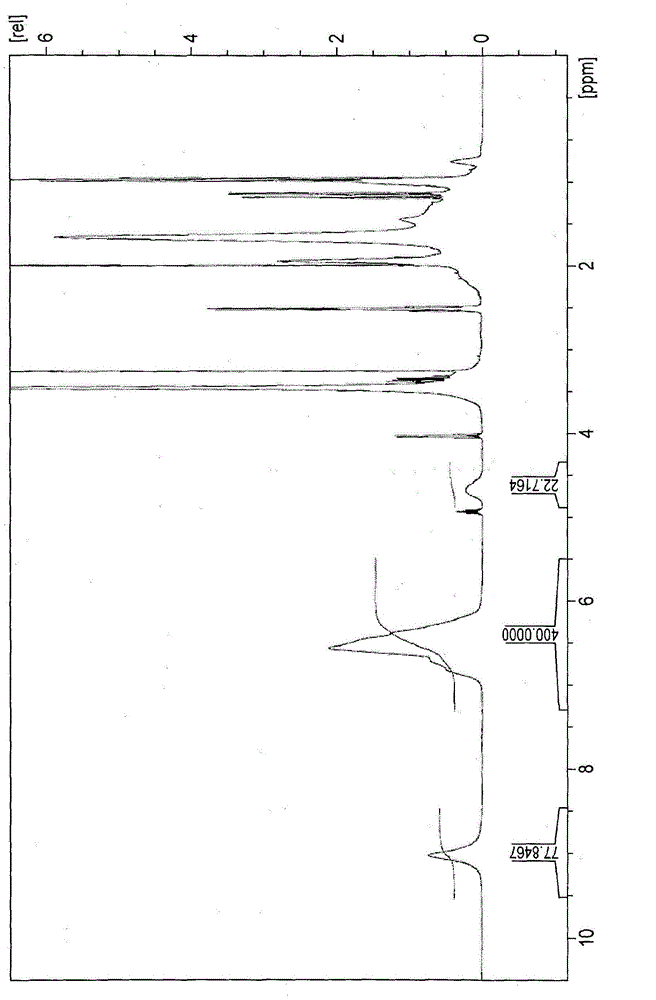
[0601] Poly(p-hydroxystyrene) (VP-2500, manufactured by Nippon Soda Co., Ltd.) weighing 30.0 g as a polyhydroxystyrene compound was dissolved in 120 g of acetone. Thereafter, 1.32 g of 1-chloromethylnaphthalene, 2.07 g of potassium carbonate (2 equivalents to 1-chloromethylnaphthalene) and 0.56 g of sodium iodide (0.5 equivalents to 1-chloromethylnaphthalene) Add to solution and reflux for four hours. About half of the amount of acetone was distilled off by means of an evaporator, and 200 ml of ethyl acetate and then 200 ml of 1N hydrochloric acid were added thereto with stirring. The mixture thus obtained was transferred to a separatory funnel and the aqueous phase was removed. The organic phase obtained was washed with 200 ml of 1N hydrochloric acid and then 200 ml of distilled water. The ...
Synthetic example 1
[0612]
[0613] (Synthesis of Chloroethers)
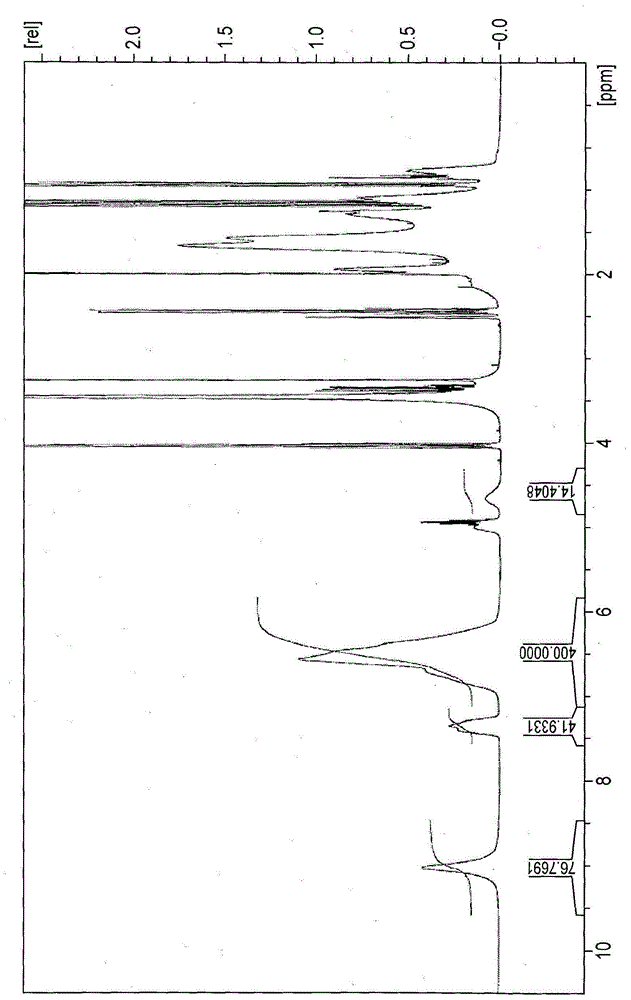
[0614] First, 20.0 g of adamantane-1-carbaldehyde, 34.35 g of cyclohexaneethanol, 1.41 g of camphorsulfonic acid and 100 ml of heptane were placed in a 300 ml in a round bottom flask and reflux for eight hours. The mixture was cooled to room temperature, and 3.1 g of triethylamine was added thereto and stirred. The organic phase thus obtained was washed twice with saturated sodium bicarbonate water and once with distilled water. Heptane and unreacted cyclohexaneethanol were removed under vacuum heating to obtain Compound 1 shown below as an acetal compound.
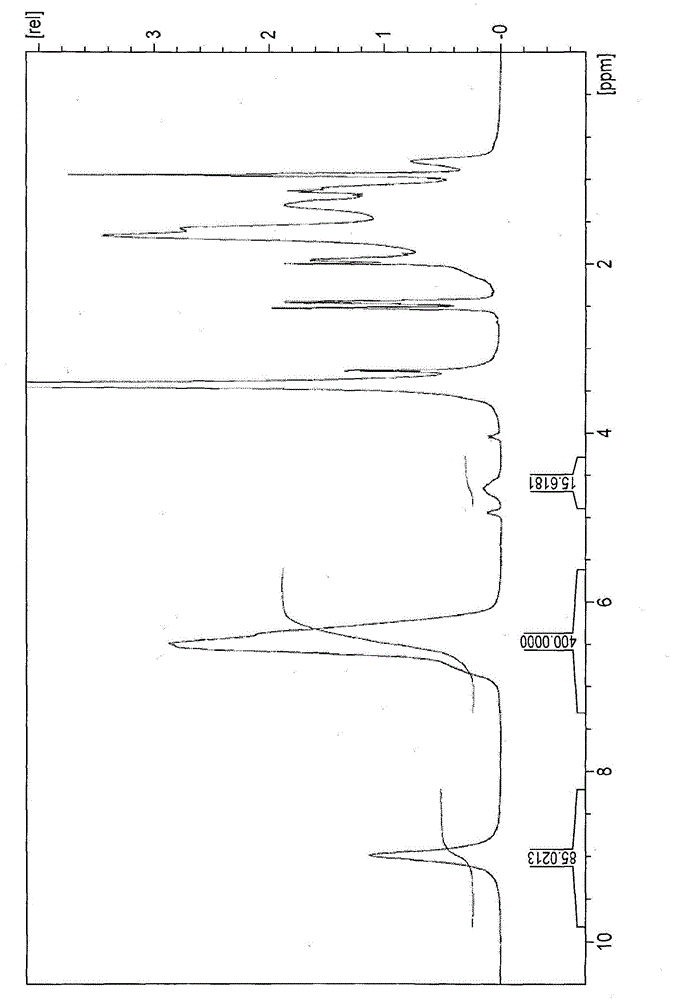
[0615] Subsequently, 11.47 g of acetyl chloride was added to the entire amount of the obtained Compound 1, and stirred in a 45° C. water bath for four hours. The mixture was cooled to room temperature, and unreacted acetyl chloride was removed in vacuo. Thus, a liquid containing Compound Cl-1 shown below as a chloroether compound was obtained. pass 1 H-NMR analysis fin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com