Synthetic method of 7-denitrified-7-substituted guanosine
A technology of guanine nucleoside and guanine nucleotide, which is applied in the fields of chemical synthesis and biochemistry, can solve the problems of expensive synthesis methods, complexity, and high price of nucleosides containing substituents, and achieve easy-to-obtain and simple raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
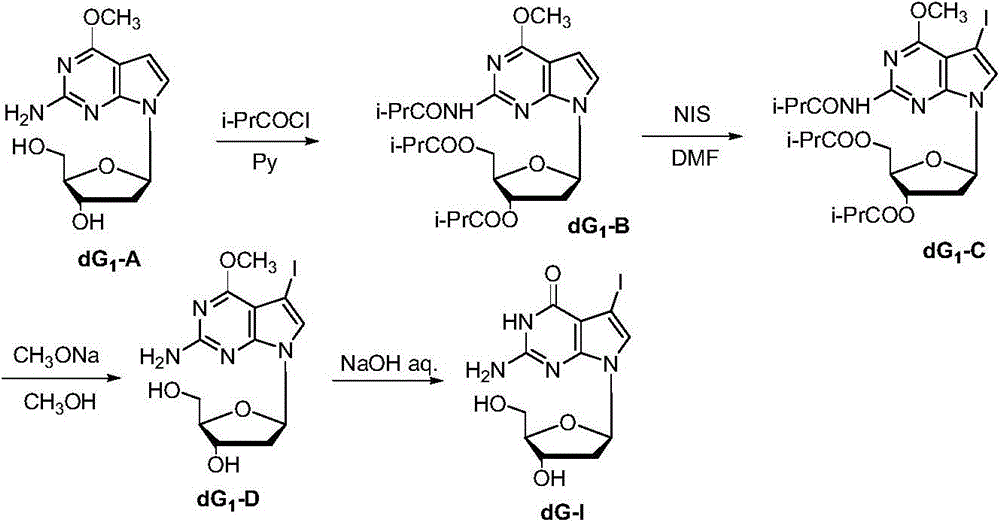
[0042] One of the synthetic methods of embodiment 1,7-deaza-7-iodo-2'-deoxyguanosine dG-I
[0043] The synthesis schematic diagram of dG-I in this embodiment is as follows figure 1 As shown, the specific synthetic method comprises the following steps respectively:
[0044] step one,
[0045]
[0046] Compound dG 1 -A (0.20g; 0.714mmol) was dissolved in anhydrous pyridine, pivaloyl chloride (0.75mL; 7.14mmol) was slowly added dropwise at 0°C, stirred at 0°C for 1h, then 2ml of methanol was added, stirred for 10min, and the solvent was spun out , adding ethyl acetate (200ml) and saturated sodium bicarbonate solution (50ml) for extraction, separating the organic phase, adding saturated sodium bicarbonate solution and saturated brine successively for washing, drying over anhydrous sodium sulfate, spinning off the solvent, and silica gel column chromatography [V (ethyl acetate): V (petroleum ether) = 1: 1] to obtain 0.39 g of white solid, namely compound dG 1 -B, yield 92%...
Embodiment 2
[0056] Embodiment 2, the second synthetic method of 7-deaza-7-iodo-2'-deoxyguanosine dG-I
[0057] The synthesis schematic diagram of dG-I in this embodiment is as follows figure 2 As shown, the specific synthetic method comprises the following steps respectively:
[0058] step one,
[0059]
[0060] After Sm-1 (27.3g, 138mmol) was added to 70mL of water, 3.0mL of concentrated hydrochloric acid was added and stirred at 90°C for 0.5h. After cooling to room temperature, sodium acetate (13.6g, 165mmol) was added and stirred, and Sm-2 ( 20.0g, 159mmol) and sodium acetate (7.0g, 85.4mmol) were dissolved in 150mL water and added to the reaction, stirred at 80°C for 2h, then moved to zero°C and stirred for 1.5h, filtered, and washed with ice water and acetone, It was sucked dry to obtain 15.4 g, and the yield was 74%. 1 H NMR (400MHz, DMSO): δ=10.94(s, 1H), 10.35(s, 1H), 6.58(dd, J=3.4, 2.2Hz, 1H), 6.15(dd, J=3.4, 2.1Hz, 1H ), 6.09(s, 2H).
[0061] Step two,
[0062]
...
Embodiment 3
[0079] The synthetic method of embodiment 3,7-deaza-7-iodine-guanosine G-I
[0080] The synthesizing schematic diagram of G-I in the present embodiment is as image 3 As shown, the specific synthetic method comprises the following steps respectively:
[0081] step one,
[0082]
[0083] G008 (1.5g, 4.0mmol) and ammonium sulfate (15mg, 0.11mmol) were refluxed in hexamethyldisilazane (15mL, 72.76mmol) for 20h under the protection of argon, and 40mL of dichloro Ethane, add G-I-O (6.0mmol) and TMSOTf (1.25mL, 6.47mmol) and stir at room temperature until clarified, then stir at 50 degrees Celsius for 24h, add 60mL DCM, and wash with 30mL saturated sodium bicarbonate and saturated brine, spin After removing the organic phase, 1.6 g of compound G-I-B was obtained by column chromatography. 1H NMR (600MHz, DMSO): δ=10.29(s, 1H), 8.02(s, 1H), 7.91-7.85(m, 6H), 7.64-7.58(m, 3H), 7.46-7.39(m, 6H) , 6.48(d, J=3.9Hz, 1H), 6.41(t, J=6.1, 6.1Hz, 1H), 6.32(dd, J=6.0, 4.0Hz, 1H), 4.82(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com