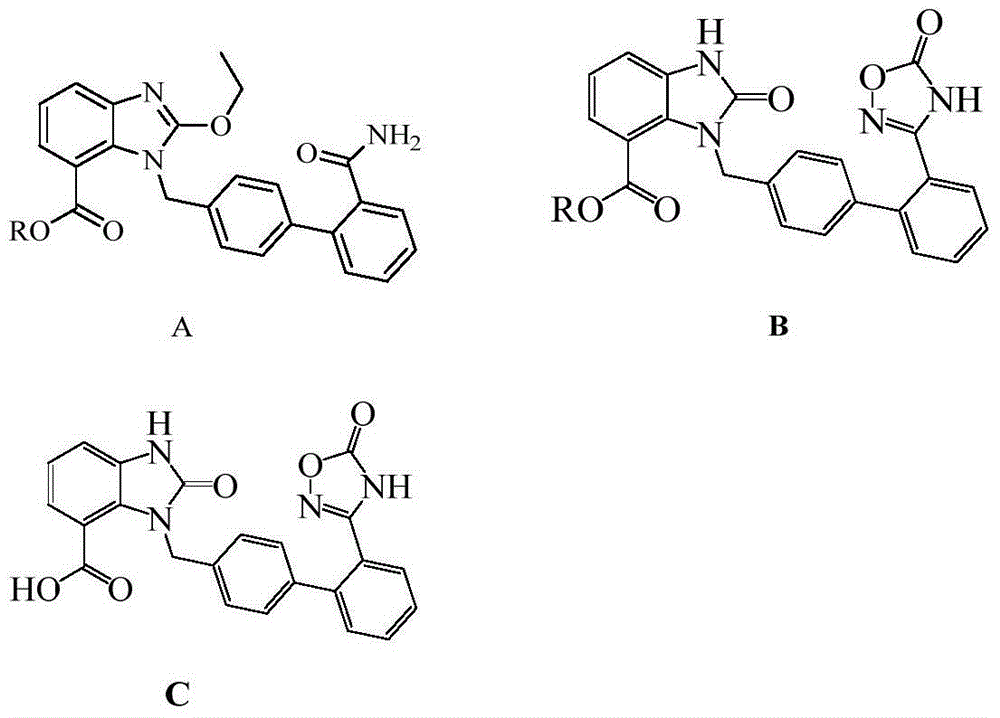
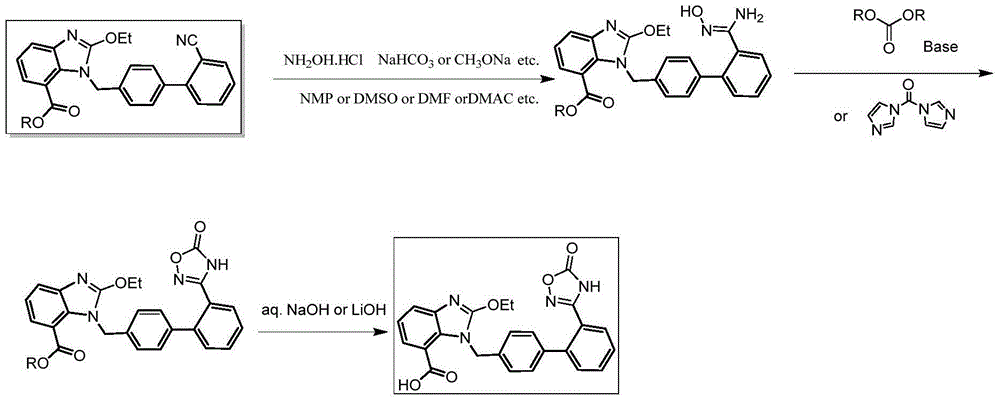
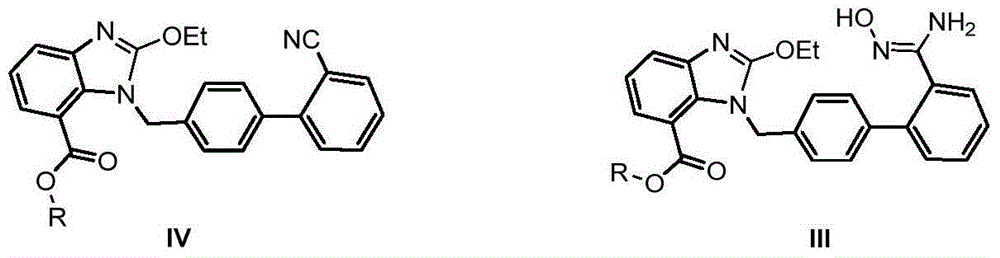
Industrial production method of azilsartan
A production method, the technology of candesartan, which is applied in the field of pharmaceutical production, can solve the problems that the cost and safety are not suitable for industrial production, cannot reach the medicinal level, and cannot be industrialized production, so as to achieve high economic value and social benefits, and increase the yield , The effect of reducing impurity B
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The specific operation steps of producing Azilsartan are as follows:
[0030] 1. Formula III compound preparation:
[0031]
[0032] Add 4.0kg dimethyl sulfoxide, 2.0kg sodium bicarbonate, 1.4kg hydroxylamine hydrochloride, and raise the temperature to about 42±2°C. Stir the reaction for about 2 hours, cool down to 25±5°C, centrifuge, wash the filter cake with 1.4kg dimethyl sulfoxide, discard the filter cake, vacuum pump the filtrate directly into the reactor, heat up to 45±2°C and add 1.0kg candesartan C6 For alkyl esters (compounds represented by formula IV), heat up to 92±2°C and keep warm for 12 hours. HPLC confirms that the raw material is less than 1.0%. Cool down to 40°C and add 3.0kg of tap water dropwise, stop dripping until turbidity occurs, cool down in a circulating ice-salt bath (cooling in stages, takes 1hr) to 10°C, keep stirring for 2hrs, centrifuge to get wet products, return to the kettle for beating once ( 1.8Kg water), centrifuged and dried to ...
Embodiment 2
[0041] The specific operation steps of producing Azilsartan are as follows:
[0042] 1. Formula III compound preparation:
[0043]
[0044] Add 4.0kg of N-methylpyrrolidone, 1.0kg of anhydrous sodium carbonate, and 1.3kg of hydroxylamine hydrochloride, and raise the temperature to about 30-40°C. Stir the reaction for about 2 hours, cool down to 30±5°C, centrifuge, add 1.0kg N-methylpyrrolidone to wash the filter cake, discard the filter cake, vacuum pump the filtrate directly into the reactor, heat up to 45±2°C and add 1.0kg candesartan C6 alkyl esters (compounds represented by formula IV), heated up to 95±5°C and incubated for 20 hours, HPLC confirmed that the raw materials were less than 1%. Cool down to 40°C and add 3.5kg of tap water dropwise, stop dripping until turbidity occurs, cool down in a circulating ice-salt bath (cooling in stages, takes 1hr) to 10°C, keep stirring for 2hrs, centrifuge to obtain wet products, return to the kettle for beating once ( 2.0kg of w...
Embodiment 3
[0053] The specific operation steps of producing Azilsartan are as follows:
[0054] 1. Formula III compound preparation:
[0055]
[0056] Add 4.0kg of N,N-dimethylacetamide, 1.35kg of potassium bicarbonate, and 1.4kg of hydroxylamine hydrochloride, and raise the temperature to about 30-40°C. Stir the reaction for about 2 hours, cool down to 25±5°C, centrifuge, add 1.0kg N,N-dimethylacetamide to wash the filter cake, discard the filter cake, vacuum pump the filtrate directly into the reactor, heat up to 55±2°C and add 1.0kg Candesartan C6 alkyl ester (compound represented by formula IV), heat up to 102±3°C and keep warm for 24hrs, and HPLC confirms that the raw material is less than 1%. Cool down to 40°C and add 3.2kg of tap water dropwise, stop dripping until turbidity occurs, cool down in a circulating ice-salt bath (cooling in stages, takes 1hr) to 10°C, keep stirring for 2hrs, centrifuge to get wet products, return to the kettle for beating once ( 2.0kg of water), ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com