Synthesis method of C13-labelled DDT (Dichlorodiphenyl Trichloroethane), DDD (Dichlorodiphenyl Dichloroethane) and DDE (Dichlorodiphenyl Dichloroethene)
A technology of 13C12-DDT and synthesis method, which is applied in the field of synthesis of DDT, DDD and DDE, and can solve problems that have not been published yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 13 C 6 -Synthesis of chlorobenzene
[0022] Add 1.00g (11.9mmol) to the 100mL reaction bottle 13 C 6 -Benzene, 8.12g (71.4mmol) hydrochloric acid and 0.38g hexadecyltributylphosphonium, stirred evenly at 10°C, slowly added 1.52g sulfuryl chloride dropwise within half an hour, and continued to react at this temperature for 10 minute. The reaction solution was cooled to room temperature, separated, dried over anhydrous magnesium sulfate, and distilled (normal pressure, 132°C) to obtain 1.21 g (86%) of the product.
Embodiment 2
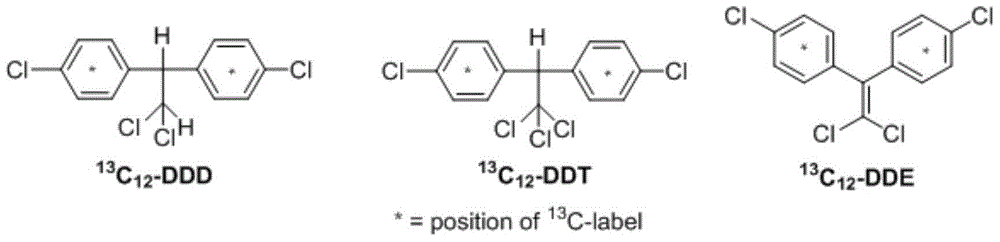
[0024] 2,2-bis(4-chlorophenyl- 13 C 6 )-1,1,1-Trichloroethane ( 13 C 12 -DDT)
[0025] Add 0.46g (3.88mmol) to the 50mL two-necked bottle 13 C 6 -Chlorobenzene, 0.32g (1.94mmol) chloral hydrate and 1.9mL sulfuric acid, stirred at 30°C for 5 hours, then added 9g of water to quench the reaction, each with 5mL of CH 2 Cl 2 The product was extracted, extracted 3 times, the organic layer was dried with anhydrous sodium sulfate, and concentrated to obtain the primary product, which was recrystallized from ethanol and dichloromethane (V:V=2:1) to obtain 0.52 g of the pure product (chemical yield 73% , isotope yield 63%). 1 H NMR (CDCl 3 ,300MHz) δ: 7.56-7.45(m,4H), 7.33-7.25(m,4H), 5.02(m,1H); HRMS(EI,positive,m / z): calcd mass for 12 C 2 13 C 12 h 9 35 Cl 4 37 Cl, [M] + : 365.9520. Found: 365.9513.
Embodiment 3
[0027] 2,2-bis(4-chlorophenyl- 13 C 6 )-1,1-dichloroethane ( 13 C 12 -DDD)
[0028] Add 0.23g (1.94mmol) to the 50mL two-necked bottle 13 C 6 -Chlorobenzene, 0.13g (0.97mmol) dichloroacetaldehyde hydrate and 0.9mL phosphoric acid, stirred at 30°C for 8 hours, then added 4.6 water to quench the reaction, each time with 5mL CH 2 Cl 2 The product was extracted, extracted 3 times, the organic layer was dried with anhydrous sodium sulfate, concentrated to obtain the primary product, and recrystallized by ethanol and dichloromethane (V:V=2:1) to obtain 0.23g of the pure product (70%, isotope yield rate 60%). 1 H NMR (CDCl 3 ,300MHz) δ: 7.46-7.19(m,8H), 6.29(d,J=7.9Hz,1H), 4.56-4.53(m,1H); HRMS(EI,positive,m / z): calcd mass for 12 C 2 13 C 12 h 10 35 Cl 337 Cl, [M] + : 331.9910. Found: 331.9914.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com