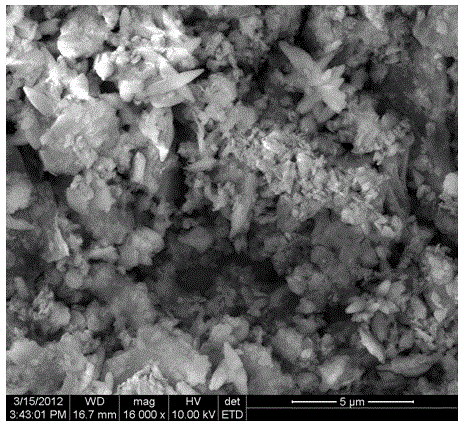
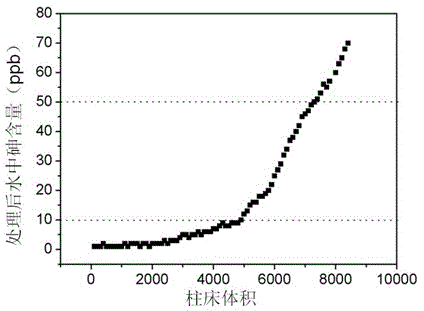
Method for preparing natural ore soil loaded nanometer arsenic removal agent
A natural ore and nanotechnology, applied in chemical instruments and methods, water/sludge/sewage treatment, adsorption water/sewage treatment, etc., can solve the problems of secondary pollution of water bodies, agglomeration and inactivation of nanomaterials, etc., and achieve equipment Less investment, simple operation, and the effect of avoiding secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Add 1088.4 grams of attapulgite to 4.5 liters of 1mol / L ferric nitrate aqueous solution (the mass of the metal salt is 1088.4g), and stir vigorously for 60 minutes to make the metal salt ions evenly and fully enter the pores of the natural mineral soil;
[0039] (2) According to the volume ratio of 1:1, add 3mol / L sodium hydroxide aqueous solution to the mixed solution in (1) until the pH value of the reaction solution is 7, and stir for 120 minutes to make it fully react;
[0040] (3) The reaction solution in (2) was allowed to stand for 360 minutes and then filtered through a membrane with a pore size of 1 micron to obtain the product;
[0041] (4) Wash the separated product in (3) with water, then dry and pulverize to obtain a powder with a particle size of less than 0.1mm;
[0042] (5) Use a granulator to granulate the powder in (4) into round pellets with a diameter of 1.0mm, dry at 110°C for 60 minutes, then sinter at 300°C for 4 hours, and finally cool natur...
Embodiment 2
[0051] (1) Add 559.8 grams of attapulgite to 3.5 liters of 0.4mol / L ferric sulfate aqueous solution (the mass of the metal salt is 559.8 g), and stir vigorously for 50 minutes to make the metal salt ions evenly and fully enter the pores of the natural mineral soil ;
[0052] (2) Add 4mol / L sodium hydroxide aqueous solution to the mixed solution in (1) at a volume ratio of 5:3 until the pH value of the reaction solution is 7.5, and stir for 150 minutes to make it fully react;
[0053] (3) The reaction solution in (2) was allowed to stand for 360 minutes and then filtered through a membrane with a pore size of 1 micron to obtain the product;
[0054] (4) Wash the separated product in (3) with water, then dry and pulverize to obtain a powder with a particle size of less than 0.1 mm;
[0055] (5) Use a granulator to granulate the powder in (4) into round pellets with a diameter of 1.1mm, dry at 130°C for 50 minutes, then sinter at 350°C for 3.5 hours, and finally cool naturally t...
Embodiment 3
[0058] (1) Add 405.5 grams of attapulgite to 5 liters of 1mol / L ferric chloride aqueous solution (the mass of the metal salt is 811.01g), and stir vigorously for 90 minutes to make the metal salt ions evenly and fully enter the pores of the natural mineral soil (2) Add 3mol / L sodium hydroxide aqueous solution to the mixed solution in (1) at a volume ratio of 1:1 until the pH value of the reaction solution is 7, and stir for 100 minutes to make it fully react;
[0059] (3) The reaction solution in (2) was allowed to stand for 360 minutes and then filtered through a membrane with a pore size of 1 micron to obtain the product;
[0060] (4) Wash the separated product in (3) with water, then dry and pulverize to obtain a powder with a particle size of less than 0.1 mm;
[0061] (5) Use a granulator to granulate the powder in (4) into round pellets with a diameter of 0.8mm, dry at 120°C for 70 minutes, then sinter at 375°C for 4.5 hours, and finally cool naturally to The natural or...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com