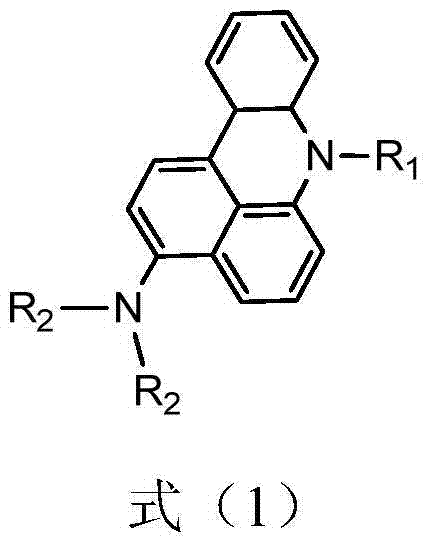
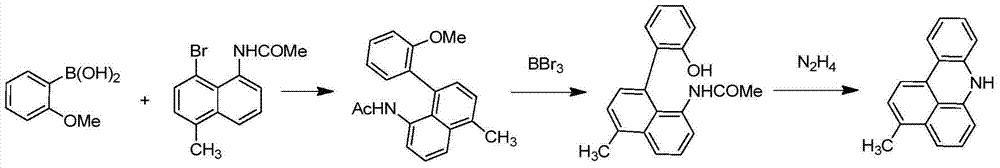
Green organic electroluminescent material and preparation method thereof
An electroluminescence and electromechanical technology, which is applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problem that the luminous efficiency of luminescent materials cannot meet the requirements of OLED, and achieves improved yield, high purity, and simple synthesis and purification. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
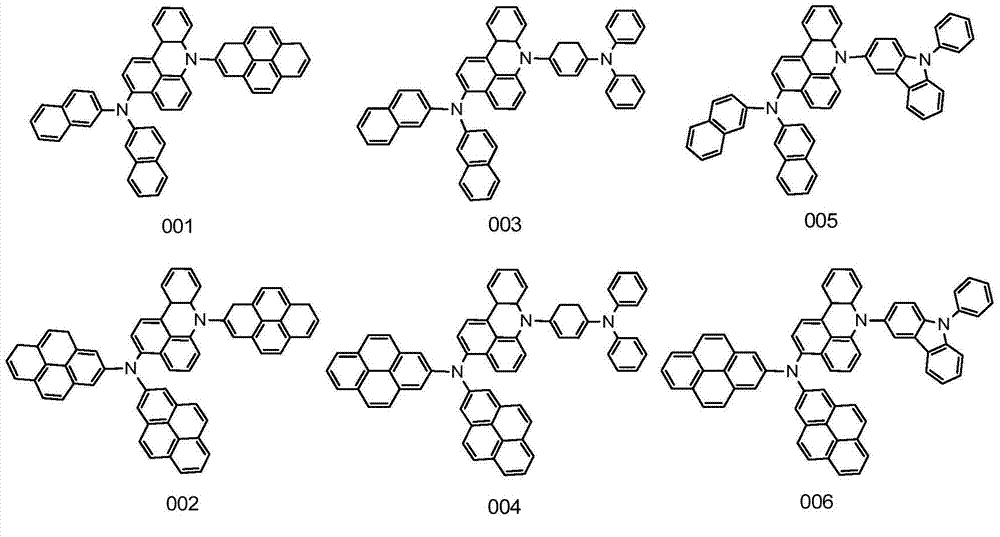
[0027] Embodiment 1: the synthesis of compound 001
[0028] Concrete synthetic route is as follows:
[0029]
[0030] Weigh 23.12g of 3-methyl-7H-benzoacridine, 42.17g of 2-bromopyrene, 16.83g of potassium tert-butoxide, 1.13g of palladium(II) acetate, and 1.02g of tri-tert-butylphosphine, and dissolve them in 250ml of toluene , and reacted at 80° C. for 10 hours under the protection of nitrogen. The reaction solution was filtered, and the crude product obtained was purified by silica gel chromatography, and the obtained solid crude product was recrystallized with toluene and dried to obtain yellow-white 7-(2-pyrenyl)-3-methyl-7H-benzoacridine Pyridine 35.81g, the yield was 83%.
[0031] Under the protection of argon, 7-(2-pyrenyl)-3-methyl-7H-benzoacridine 35.81g, ammonia water 36.75g, palladium 0.76g, titanium dioxide photocatalyst, to obtain 7-(2-pyrenyl) -7H-Benzacridin-3-amine 30.51g, yield 85%.
[0032] Under the protection of nitrogen, 15.14g of 7-(2-pyrenyl)-7H-...
Embodiment 2
[0033]Embodiment 2: the synthesis of compound 002
[0034] Concrete synthetic route is as follows:
[0035]
[0036] Weigh 23.12g of 3-methyl-7H-benzoacridine, 42.17g of 2-bromopyrene, 16.83g of potassium tert-butoxide, 1.13g of palladium(II) acetate, and 1.02g of tri-tert-butylphosphine, and dissolve them in 250ml of toluene , and reacted at 80° C. for 10 hours under the protection of nitrogen. The reaction solution was filtered, and the crude product obtained was purified by silica gel chromatography, and the obtained solid crude product was recrystallized with toluene and dried to obtain yellow-white 7-(2-pyrenyl)-3-methyl-7H-benzoacridine Pyridine 35.81g, the yield was 83%.
[0037] Under the protection of argon, 7-(2-pyrenyl)-3-methyl-7H-benzoacridine 35.81g, ammonia water 36.75g, palladium 0.76g, titanium dioxide photocatalyst, to obtain 7-(2-pyrenyl) -7H-Benzacridin-3-amine 30.51g, yield 85%.
[0038] Under nitrogen protection, 15.14g of 7-(2-pyrenyl)-7H-benzoacr...
Embodiment 3
[0039] Embodiment 3: the synthesis of compound 003
[0040] Concrete synthetic route is as follows:
[0041]
[0042] Weigh 23.12g of 3-methyl-7H-benzoacridine, 58.35g of 4-bromotriphenylamine, 21.54g of potassium tert-butoxide, 1.74g of palladium (II) acetate, 1.56g of tri-tert-butylphosphine, and use 250ml of toluene Dissolve and react at 85°C for 13 hours under the protection of nitrogen. The reaction solution was filtered, and the obtained crude product was purified by silica gel chromatography, and then the obtained solid crude product was recrystallized with toluene and dried to obtain the yellow-white intermediate 7-(4-triphenylamino)-3-methyl-7H- Benzacridine 40.34g, yield 85%.
[0043] Under the protection of argon, 7-(2-pyrenyl)-3-methyl-7H-benzoacridine 40.34g, ammonia water 37.88g, palladium 0.78g, titanium dioxide photocatalyst, to obtain 7-(2-pyrenyl) -7H-Benzacridin-3-amine 33.45 g, yield 85%.
[0044] Under nitrogen protection, 16.72g of 7-(4-triphenylam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com