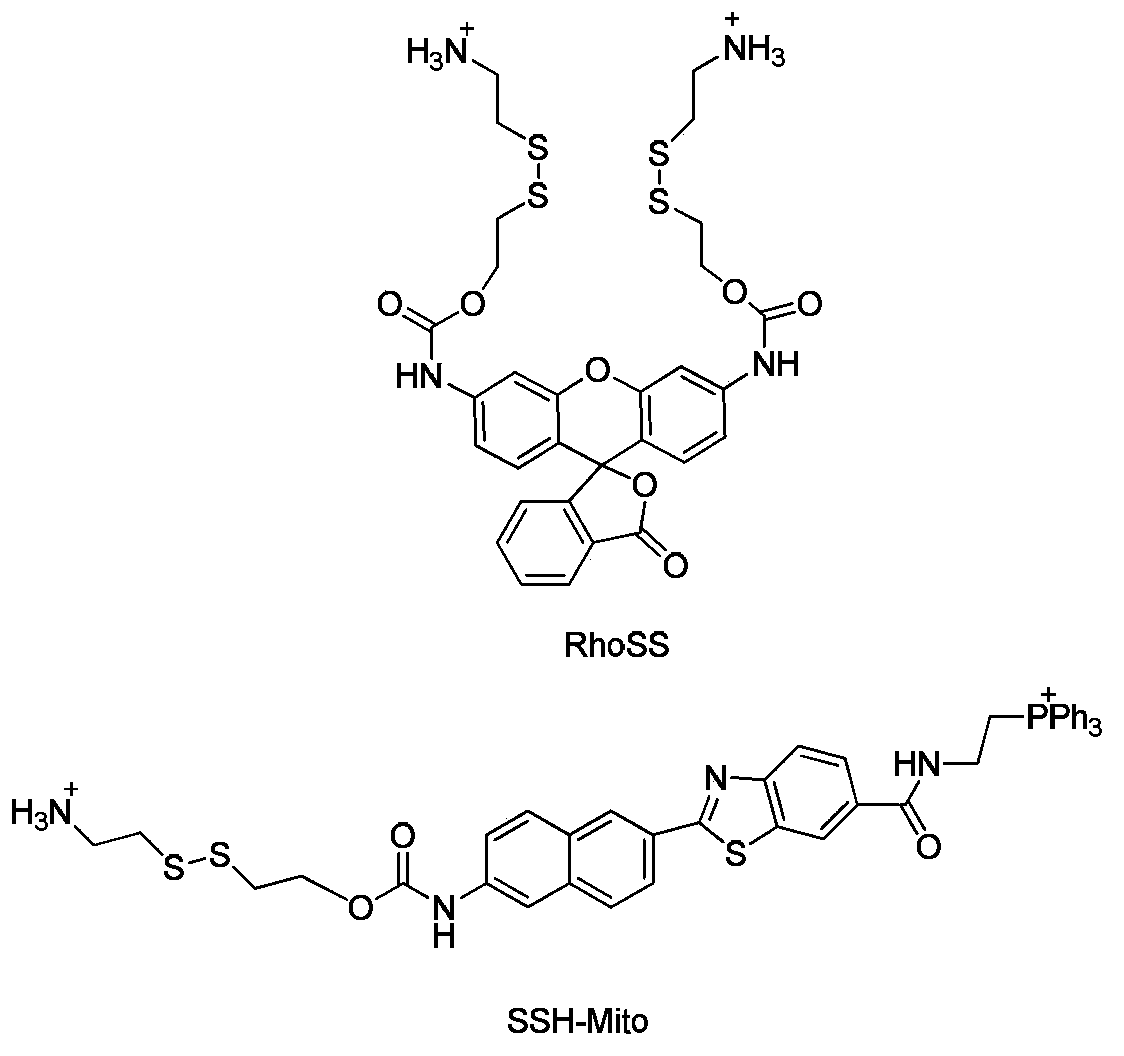
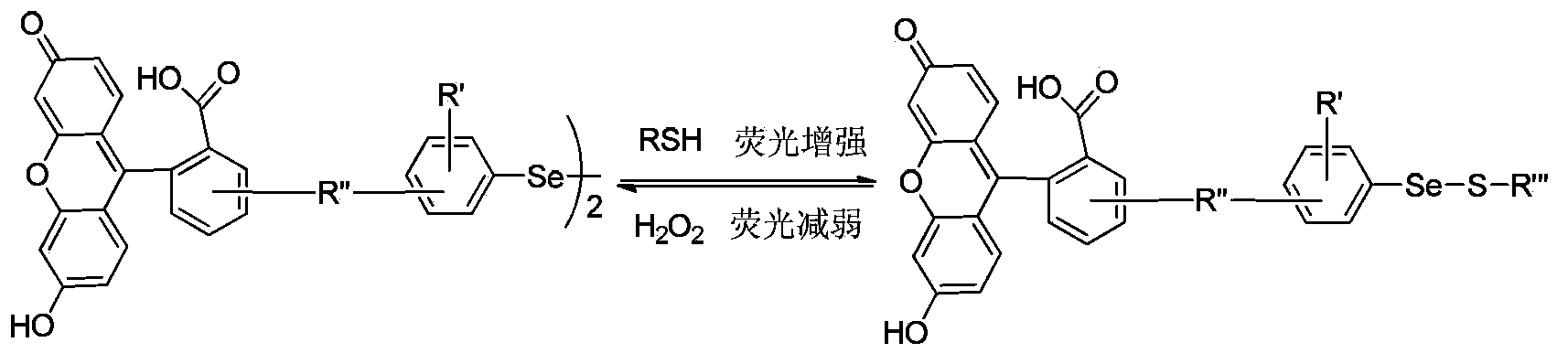
Fluorescent probe and application thereof in dynamic detection of mercaptan
A fluorescent probe and dynamic detection technology, applied in the field of fluorescent probes, can solve the problems of inability to accurately provide thiol concentration changes, inability to be dynamic, and lack of application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 (synthesis of probe):
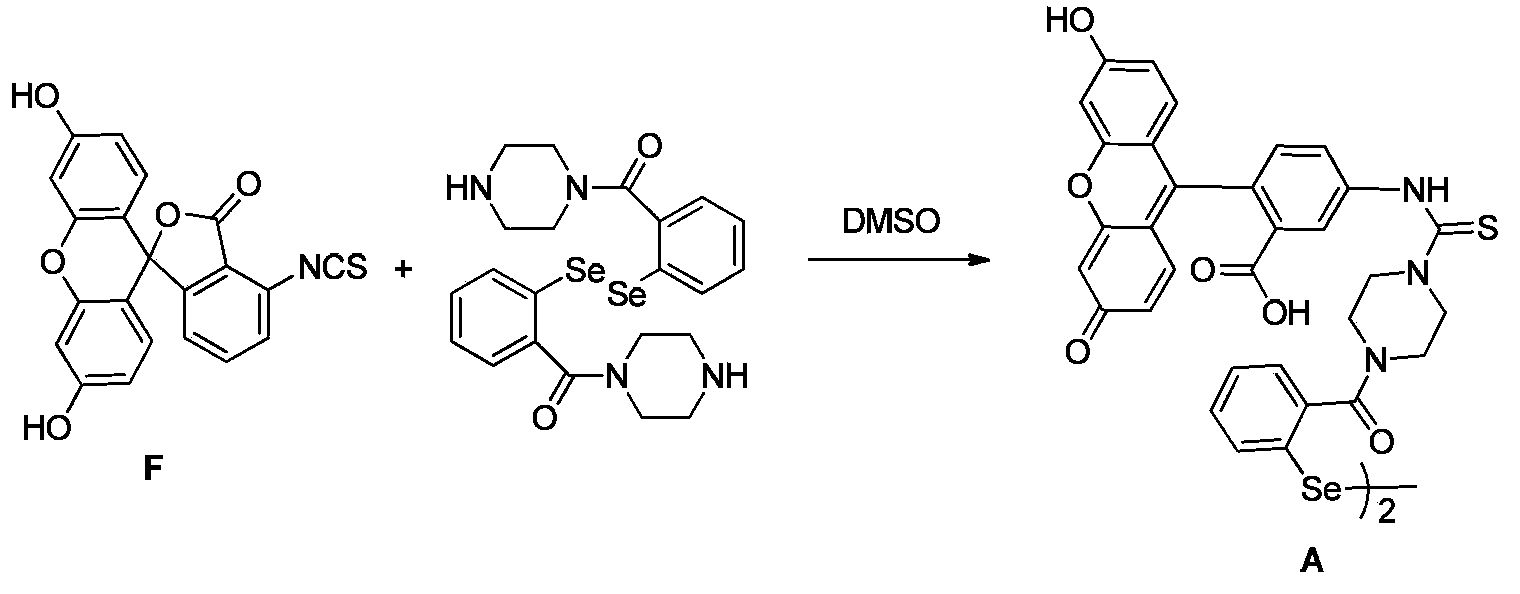
[0059] like image 3 As shown, the structure of the probe compound used in the examples is represented by the code A, and the fluorescein precursor used in the synthesis of the probe compound is represented by the code F.
[0060] Synthesis of A: 0.6g of F (available for purchase) and 2.5g of 2-piperazinoyl-1-phenylselenide were dissolved in 25ml of anhydrous dimethyl sulfoxide, and stirred at room temperature for 85 hours. The solvent was evaporated under vacuum, and the obtained solid was purified by column chromatography to obtain the target compound A.
[0061] 1 H NMR (400MHz, d 6 -DMSO)δ(ppm):11.97(br,1H),10.13(s,2H),9.77(s,1H),7.98-7.97(d,1H),7.80-7.77(m,2H),7.43-7.42 (m,3H),7.20-7.18(m,1H),6.68-6.67(d,2H),6.59-6.58(m,3H),4.05-4.00(b r,4H),3.78(br,2H),3.43 (br,2H). 13 C NMR (100MHz, d 6 -DMSO)δ(ppm):181.50,181.37,171.88,168.49,167.92,159.43,159.26,151.80,147.81,142.67,142.54,136.29,131.89,131.73,131.63,130.64,129.25,128...
Embodiment 2
[0063] Embodiment 2 (A is to the selectivity of thiol):
[0064] The pH was controlled with PBS buffer solution. Add 2.0μM A to a 10ml colorimetric tube, then add 20mMPBS, dilute the volume to 10ml with ultrapure water, shake the solution, add the above working solution into a fluorescent dish to measure the fluorescence spectrum. Fluorescence intensity changes with pH as Figure 4 shown. Figure 4 It shows that there is no obvious change in the fluorescence intensity of A near the physiological pH, that is, A can be used to detect thiols in a system with a pH of 7.0-10.0.
[0065] In order to simulate physiological conditions as much as possible, the following experiments were carried out under the condition of PH=7.4 (PBS buffer solution, the concentration is 20mM).
[0066] Add 2.0μM probe to a 10ml colorimetric tube, then add 20mM PBS pH7.4, add ultrapure water to 10ml, shake well, and then add various analytes (see the attached figure for the amount of each analyte, bl...
Embodiment 3
[0067] Embodiment 3 (A is to the quantitative detection of mercaptan):
[0068] Add 2.0μM A to a 10ml colorimetric tube, then add 20mM PBS pH7.4, add ultrapure water to 10ml, shake well, and then add different concentrations of thiols. Shake the solution evenly, pour the working solution into a fluorescent dish to measure the fluorescence spectrum, take the maximum value of each fluorescence spectrum, and input it into the software OriginPro8.0 to obtain a linear working curve.
[0069] Thiol concentration after constant volume: 0, 0.2, 0.4, 0.8, 1.0μM.
[0070] Figure 6 (a) shows the change of fluorescence intensity of the system with the change of thiol concentration, indicating that the fluorescence of the system is obviously enhanced with the increase of thiol concentration; Figure 6 (b) represents the linear fitting curve of the fluorescence intensity changing with the thiol concentration, and the linear regression constant of the linear fitting curve is 0.99488, indi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com