The preparation method of polyaniline
A polyaniline and aniline technology, applied in the field of electrode material preparation, can solve the problems of complex polyaniline preparation process, low product polymerization degree, narrow molecular weight distribution, etc., and achieve the effects of low cost, high electrochemical activity, and narrow molecular weight distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
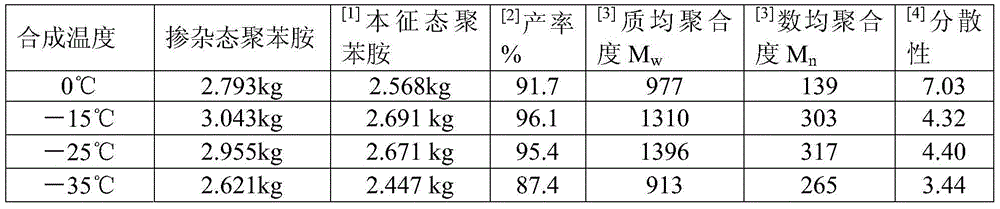
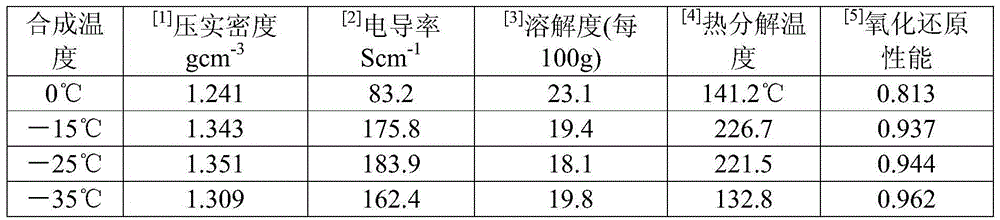
[0027] Embodiment 1 (the influence of temperature of reaction)
[0028] Taking the 70L reactor as an example, the effective reaction volume is 50L. First add 35L of 3mol / L hydrochloric acid to the reaction kettle, then add 7kg of lithium chloride, stir until it is completely dissolved and cool down to 0°C, then add 2.8kg of aniline, stir for 30min, add 1.05kg of manganese dioxide after cooling down to 0°C, Stir the reaction for 2 hours, then add dropwise 10L of ammonium persulfate solution with a concentration of 620g / L (containing 6.2kg of ammonium persulfate in total, the molar ratio of manganese dioxide to ammonium persulfate is 4:9, the molar ratio of the total amount of oxidant to aniline is 1.3︰1), the ammonium persulfate solution is also used to control the temperature below 5°C in an ice-water bath. The ammonium persulfate solution was added dropwise at a rate of 1 L / h, and the drop was completed in 10 hours, and then the stirring reaction was continued for 12 hours, ...
Embodiment 2
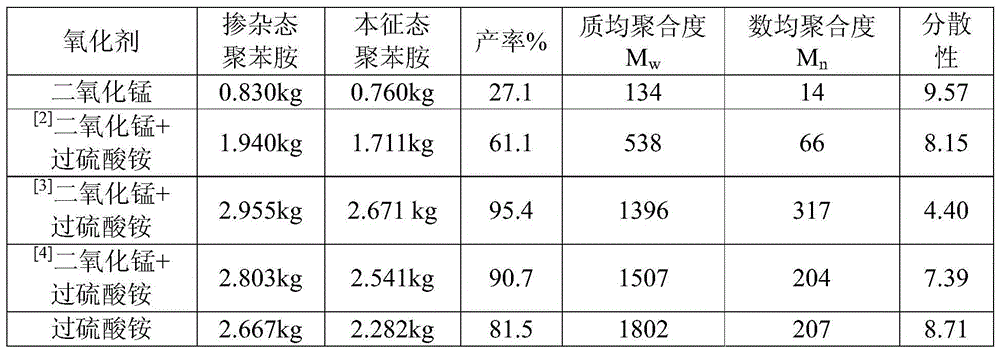
[0044] Embodiment 2 (the influence of oxidizing agent)
[0045] Taking the 70L reactor as an example, the effective reaction volume is 50L. Add 35L of 3mol / L hydrochloric acid into the reaction kettle, then add 7kg of lithium chloride, stir until it is completely dissolved and cool down to 0°C, then add 2.8kg of aniline, stir for 30min, cool down to 0°C, add manganese dioxide, The reaction was stirred for 2 hours, and then the ammonium persulfate solution was added dropwise, and the temperature of the ammonium persulfate solution was also controlled below 5°C with an ice-water bath. The ammonium persulfate solution was added dropwise at a rate of 1 L / h, and the drop was completed in 10 hours, and then the stirring reaction was continued for 12 hours, and the temperature of the reaction kettle was controlled at -15°C during the entire reaction process. After the reaction, use 800-mesh polypropylene filter cloth to suction filter, wash with deionized water, 50 L of water each t...
Embodiment 3
[0056] Embodiment 3 (the influence of hydrogen ion concentration)
[0057] The impact of table 5 hydrogen ion concentration on polyaniline yield and molecular weight
[0058]
[0059] It can be seen from Table 5 that when the concentration of hydrogen ions is 0.5 mol / L, due to the low concentration of hydrogen ions, aniline cations cannot be formed with aniline monomers, resulting in the aniline in the solution being in a molecular state, increasing various side reactions, and a large number of molecules State aniline was oxidized to quinones, resulting in a yield of only 41.9%, a mass-average degree of polymerization of 624, and a high dispersity of 10.23. As the hydrogen ion concentration increases to 1.5mol / L, it is enough to convert all the aniline monomers into aniline cations, and the reaction liquid system is acidic. At this time, aniline can strictly follow the 1,4-position polymerization reaction, and the side reactions are greatly reduced. , the yield increased b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| freezing point | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com