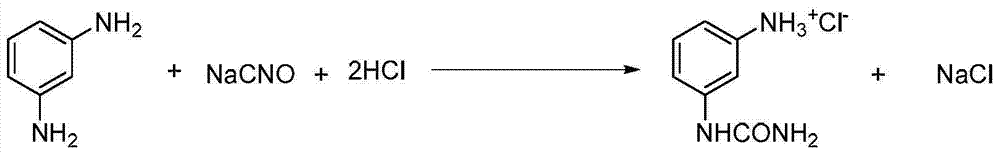
Clean production process of m-carbamidoaniline hydrochloride
A technology of ureidoaniline and clean production, which is applied in the field of clean production process of high-purity m-ureidoaniline hydrochloride, which can solve the problems of low solid content of hydrochloride, unstable dye quality, and large content detection deviation. Achieve the effects of increasing solid content, preventing the formation of diurea, and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Put 1600L of pure water in the reaction pot and start stirring. Drop into industrial sodium chloride 300kg in reaction pot. Close the equipment, open the vacuum to inhale 220kg of m-phenylenediamine, add 1.1kg of sodium nitrate, add 30% hydrochloric acid to adjust the pH value to below 0.8, after the material is completely dissolved, after cooling down to 0°C, open the automatic feeding device and start adding sodium cyanate Powder, at the same time open the hydrochloric acid automatic dripping system to add hydrochloric acid dropwise. The online system controls pH=0.8-1.0, adding 50% of the total amount of sodium cyanate within 2 hours through the automatic feeding device, and controlling the pH=1.0-1.3, adding 23% of the total amount of sodium cyanate within 1 hour , control the pH=1.1-1.4, add 25% of the total amount of sodium cyanate within 2 hours, control the pH=1.2-1.5, add 2% of the total amount of sodium cyanate within 1 hour, after adding , after half an hou...
Embodiment 2
[0034] Collect the filtrate of Example 1 and lower the temperature to 5°C to precipitate diurea. After filtering to remove impurities, collect 2800 kg of brine, take 1500 kg of it, add 400 kg of water to adjust the concentration of brine, and put it into the reaction pot. Seal the equipment and open the vacuum to inhale m-phenylenediamine. 220kg, add 6 kilograms of sodium nitrate, add sodium cyanate and hydrochloric acid by the method for embodiment 1, this process consumes hydrochloric acid 573kg, sodium cyanate 139kg.
[0035] After the reaction, heat up to 100°C to dissolve and then cool down to 20°C for recrystallization. After the filter cake was pressed dry, the filter cake was 374kg, the HPLC purity of the filter cake was 98.64%, the solid content of the filter cake was 80.0%, and the yield was 97.36%.
Embodiment 3
[0037] After the filtrate of Example 2 is collected, the temperature is lowered to 0°C to precipitate diurea, and after filtering to remove impurities, 2800 kg of brine is collected, 1400 kg of which is added to 500 kg of water to adjust the concentration of brine, and then put into a closed device in a reaction pot, and 220 kg of m-phenylenediamine is inhaled by vacuum. , suck 3 kilograms of nitric acid, add sodium cyanate and hydrochloric acid by the method for embodiment 1, this process consumes hydrochloric acid 569kg, sodium cyanate 140kg.
[0038] After the reaction, heat up to 100°C to dissolve and then cool down to 40°C for recrystallization. After the filter cake was pressed dry, the filter cake was 378kg, the HPLC purity of the filter cake was 98.63%, the solid content of the filter cake was 77.9%, and the yield was 95.78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com