Application of alanine as vaccine adjuvant
A vaccine adjuvant and alanine technology, applied in the field of biomedicine, can solve problems such as adjuvant disease, and achieve the effect of not causing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The cultivation and processing of embodiment 1 Edwardsiella tarda EIB202 bacterial strain
[0019] Pick the single clone of Edwardsiella tarda EIB202 on the Tryptone Soy Broth (TSB) plate in a TSB test tube, culture at 30°C for 24 hours, transfer to 100mL TSB medium at a ratio of 1:100 to expand the culture. When the OD600 value reached 1.0, the bacteria were collected by centrifugation, washed twice with normal saline, and the bacteria were suspended with normal saline to make the OD600 1.0 (the number of bacteria was about 109 bacteria / ml), which was used to challenge the experimental mice.
[0020] At the same time, take another part of the bacteria, add 0.1% formaldehyde, and incubate at 30°C for 24 hours to inactivate the bacteria. Then wash with normal saline for 3 times, suspend with normal saline to make OD600 1.0 (about 109cfu / mL), and then dilute 10 times with normal saline (about 108cfu / mL) to get the inactivated bacterial vaccine, which is used for experimen...
Embodiment 2
[0021] The immunization of embodiment 2 mice
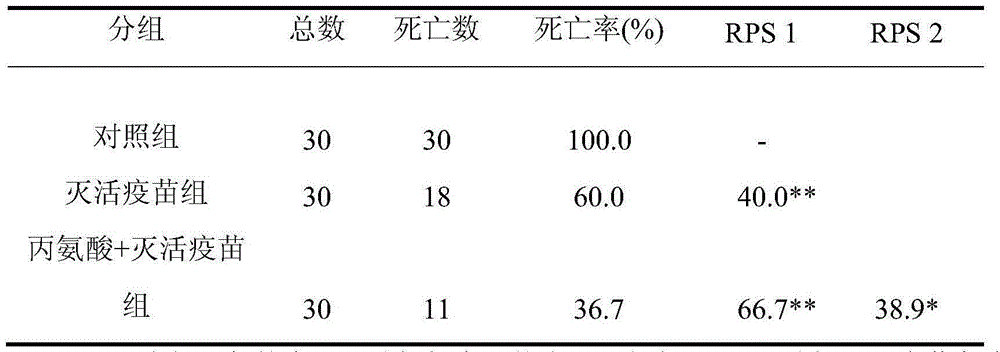
[0022] Ninety 4-week-old Kunming mice (average body weight 25g) were randomly divided into 3 groups: control group, inactivated bacteria immunization group and inactivated bacteria + alanine immunization group. Each mouse in the control group was intraperitoneally injected with 100 μL of normal saline; each mouse in the inactivated bacteria immunization group was injected intraperitoneally with 100 μL of the prepared inactivated bacteria vaccine (about 10 7 bacteria); in the inactivated bacteria + alanine immunization group, each mouse was intraperitoneally injected with the mixture of 100 μL of the prepared inactivated bacteria vaccine and 120 μmoL of alanine. Two consecutive immunizations with an interval of 7 days. Seven days after the last immunization, the virus was challenged with EIB202 at a dose of 3×10 8 CFU / only, observed for 15 days, and counted the death situation of the mice. Calculate the relative immune protectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com