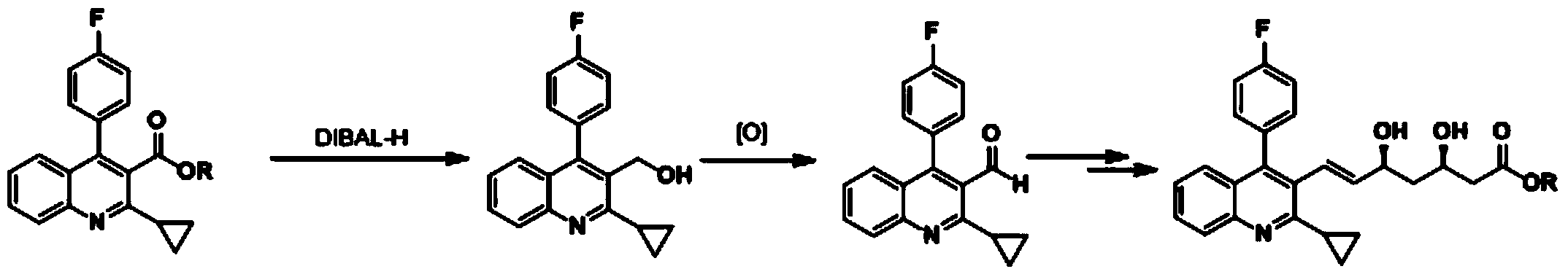
Method for preparing pitavastatin calcium
A technology of pitavastatin calcium and its compounds, which is applied in the field of preparation of pitavastatin calcium, can solve the problems of Mitsunobu reaction being difficult to scale up, low yield, sulfide environmental hazards, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: A method for preparing pitavastatin calcium, characterized in that the specific preparation steps are as follows:
[0049] (1) Synthesis of methyl 2-cyclopropyl-4-(4-fluorophenyl)quinoline-3-carboxylate (compound 3)
[0050] Add 215g (1mol) of (2-aminophenyl)(4-fluorophenyl)methanone (compound 2), 2.1L (10mL / g) of acetic acid, and 3-cyclopropyl-3 to a 5L four-necked flask. -170g (1.2mol) of methyl oxopropionate and 4.8g (0.05mol) of concentrated sulfuric acid, heated to 90-100°C. After the TLC reaction is over, the temperature is lowered to 0-10°C. The system was added to 2.1L (10mL / g) of 30% ammonia water to quench the reaction, and 2.1L (10mL / g) of dichloromethane was added to extract the product. The organic phase was washed with 2.1L saturated sodium chloride aqueous solution and concentrated to dryness to obtain the crude product. 1.05L of petroleum ether (5mL / g) was added to recrystallize to obtain the solid product 2-cyclopropyl-4-(4-fluorophenyl)quine. ...
Embodiment 2
[0063] Example 2: A method for preparing pitavastatin calcium, characterized in that the specific preparation steps are as follows:
[0064] (1) Synthesis of ethyl 2-cyclopropyl-4-(4-fluorophenyl)quinoline-3-carboxylate (compound 3)
[0065] Add 215g (1mol) of (2-aminophenyl)(4-fluorophenyl)methanone (compound 2) and 2.1L (10mL / g) of N,N-dimethylformamide to a 5L four-necked flask. , 156 g (1.0 mol) of ethyl 3-cyclopropyl-3-oxopropionate and 9.8 g (0.10 mol) of concentrated sulfuric acid, the temperature is increased from 90 to 100°C. After the TLC reaction is over, the temperature is lowered to 0-10°C. The system was added to 2.1L (10mL / g) of 30% sodium hydroxide, and 2.1L (10mL / g) of dichloromethane was added to extract the product. The organic phase was washed with 2.1 L saturated aqueous sodium chloride solution and concentrated to dryness. The obtained crude product was recrystallized by adding 1.05L of cyclohexane (5mL / g) to obtain solid ethyl 2-cyclopropyl-4-(4-fluorophen...
Embodiment 3
[0078] Embodiment 3: A method for preparing pitavastatin calcium, characterized in that the specific preparation steps are as follows:
[0079] (1) Synthesis of methyl 2-cyclopropyl-4-(4-fluorophenyl)quinoline-3-carboxylate (compound 3)
[0080] Add 215g (1mol) of (2-aminophenyl)(4-fluorophenyl)methanone (compound 2), 1.05L (5mL / g) of propionic acid, and 3-cyclopropyl- 340g (2.0mol) of methyl 3-oxopropionate and 9.6g (0.1mol) of concentrated sulfuric acid were heated to 100°C. After the TLC reaction is over, the temperature is reduced to 0-10°C. The system was added to 2.1L (10mL / g) of 15% sodium hydroxide aqueous solution, and 1.05L (5mL / g) of dichloromethane was added to extract the product. The organic phase was washed with 2.1 L saturated aqueous sodium chloride solution and concentrated to dryness. The obtained crude product was recrystallized by adding 1.05 L of petroleum ether (5 mL / g) to obtain methyl 2-cyclopropyl-4-(4-fluorophenyl)quinoline-3-carboxylate with a yield o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com