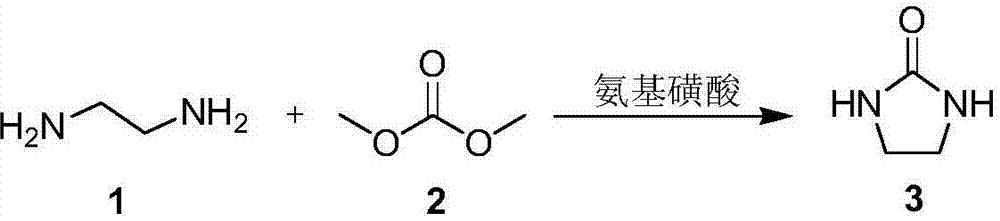
2-imidazolidone synthesis method
A technology of imidazolidinone and synthesis method, applied in the field of synthesis of 2-imidazolidinone, can solve the problems of high raw material price, high price of ethylene carbonate, use price, etc., and achieves simple and easy operation of reaction process and green synthesis method. The effect of cleanliness and less discharge of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Into a heatable reactor (with stirring device), add 1.0g sulfamic acid (0.01mol), 6mL methanol, 3.0g ethylenediamine (0.05mol), 5.4g dimethyl carbonate (0.06mol), 60 °C for 3 hours, then the temperature was raised to 160 °C, and the reaction was stopped after 15 hours of reaction. The reaction solution was naturally cooled to room temperature, filtered to remove the catalyst, and then the solvent was removed to obtain the crude product 2-imidazolidinone. Quantitative analysis was carried out by gas chromatography, and the yield was 62.1%.
[0026] NMR analysis results:
[0027] 1 H NMR (400MHz,D 2 O): δ3.47(s,4H). 13 C NMR (100MHz,D 2 O): δ40.69 (2C), 167.07 (1C).
Embodiment 2
[0029] Into a heatable reactor (with stirring device), add 1.0g sulfamic acid (0.01mol), 6mL methanol, 3.0g ethylenediamine (0.05mol), 6.3g dimethyl carbonate (0.07mol), 60 °C for 3 hours, then the temperature was raised to 160 °C, and the reaction was stopped after 15 hours of reaction. The reaction solution was naturally cooled to room temperature, filtered to remove the catalyst, and then the solvent was removed to obtain the crude product 2-imidazolidinone. Quantitative analysis was carried out by gas chromatography, and the yield was 63.4%.
Embodiment 3
[0031] Into a heatable reactor (with stirring device), add 1.0g sulfamic acid (0.01mol), 6mL methanol, 3.0g ethylenediamine (0.05mol), 7.2g dimethyl carbonate (0.08mol), 60 °C for 3 hours, then the temperature was raised to 160 °C, and the reaction was stopped after 15 hours of reaction. The reaction solution was naturally cooled to room temperature, filtered to remove the catalyst, and then the solvent was removed to obtain the crude product 2-imidazolidinone. Quantitative analysis was carried out by gas chromatography, and the yield was 63.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com