Method for adopting quantitative structure-activity relationship model to predicting soil or sediment adsorption coefficients of organic compound
An organic compound and activity relationship technology, applied in the field of ecological risk assessment and testing strategies, can solve problems such as insufficient transparency of algorithms, poor model fitting ability, and unpredictability, and achieve the effects of easy application and promotion, good fitting ability, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
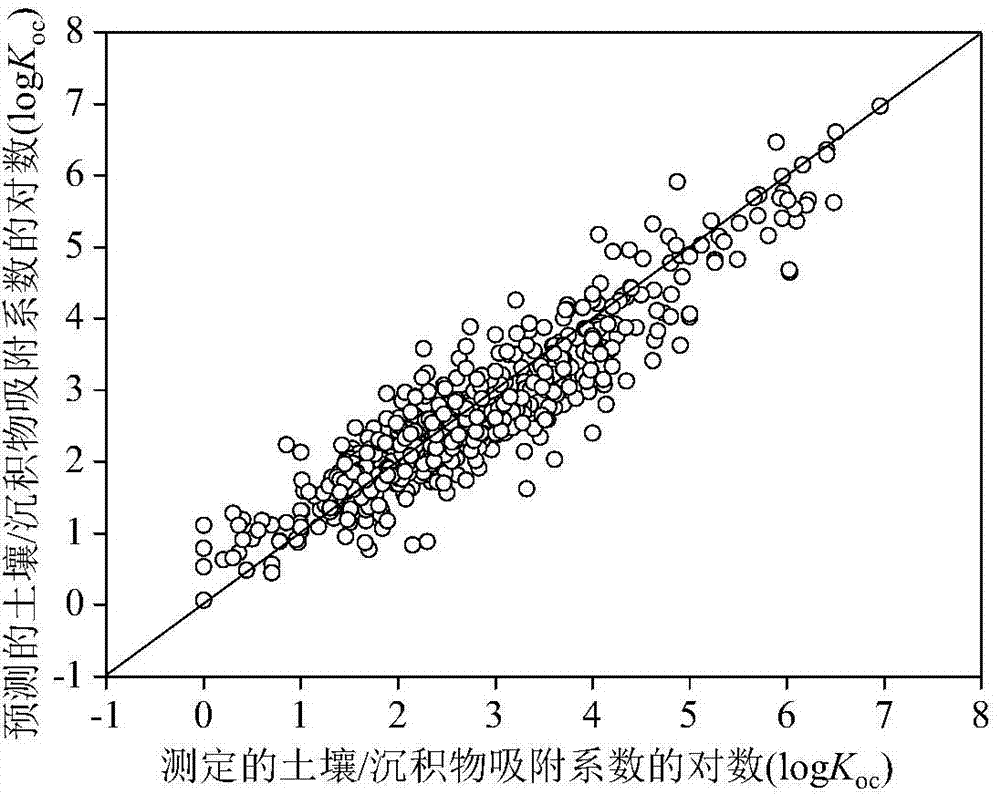
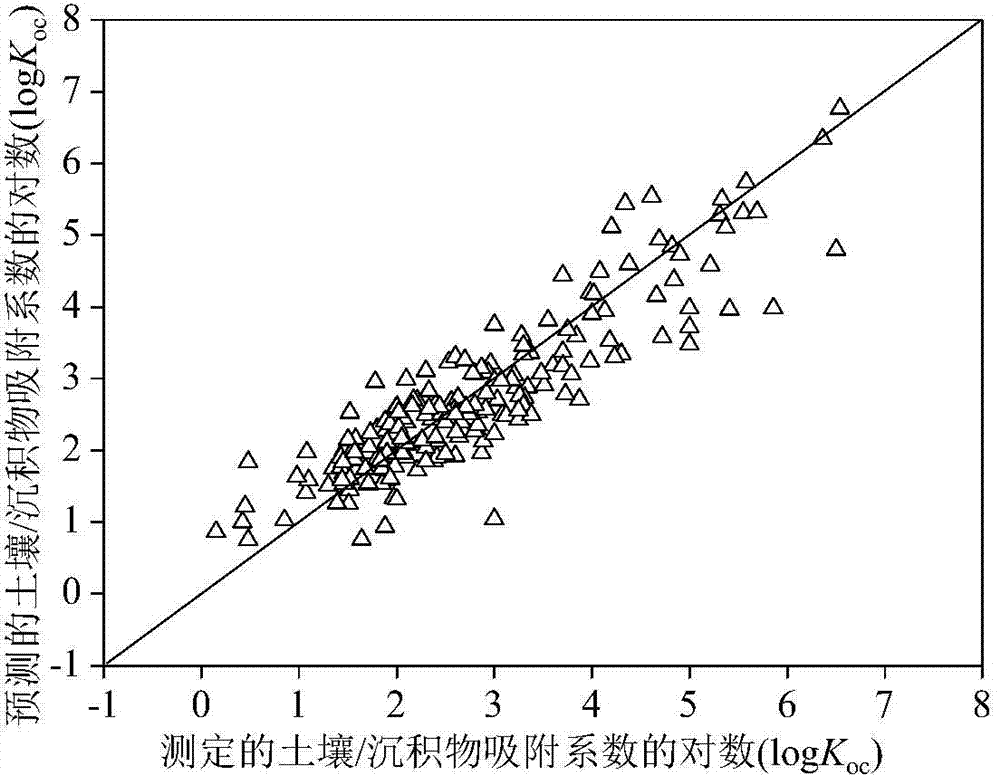
[0026] Given a compound nicotine, to predict its logK oc value. First, according to the structural information of nicotine, use the Gaussian09 software package to optimize its structure. Based on the Gaussian optimized structure, use Draogon6.0 software to calculate nHM, WiA_Dt, H_D / Dt, HATS4v, R3e+, nRCN, nR=CRX, O- The values of 061, P-117, CATS2D_05_NL, B03N-S, B08Br-Br, F02S-S, F05N-O and MLOGP2 are 0, 5.955, 156.433, 0.159,
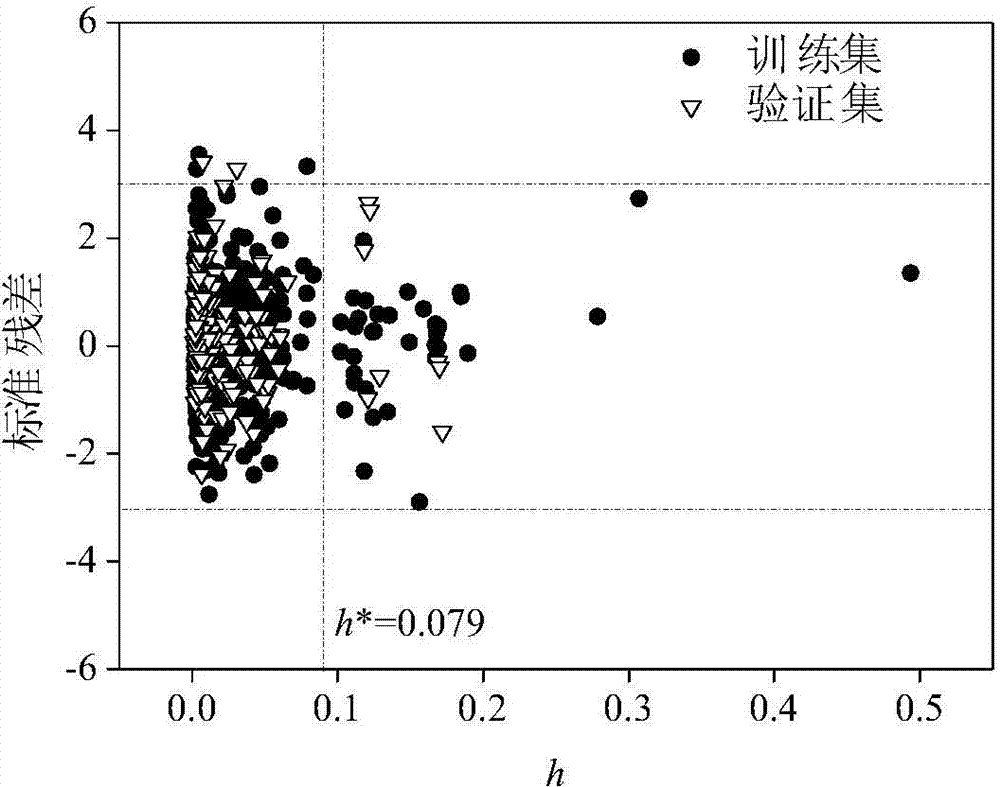
[0027] 0.069, 0, 0, 0, 0, 0, 0, 0, 0, 0, and 1.619. The h value calculated according to the formula (2) is 0.005 (oc The predicted value of 2.02, its experimentally determined logK oc The value is 2.01, and the predicted and experimental data are in good agreement.
Embodiment 2
[0029] Given a compound diethyl phthalate, to predict its logK oc value. First, according to the structural information of diethyl phthalate, use the Gaussian09 software package to optimize its structure. Based on the Gaussian optimized structure, use Draogon6.0 software to calculate nHM, WiA_Dt, H_D / Dt, HATS4v, R3e+, nRCN, nR=CRX, O-061, P-117, CATS2D_05_NL, B03N-S, B08Br-Br, F02S-S, F05N-O and MLOGP2 values are 0, 5.85, 203.902, 0.102, 0.066, 0, 0, 0 , 0, 0, 0, 1, 0, 0 and 6.641. The h value calculated according to the formula (2) is 0.003 (oc The predicted value of 2.27, its experimentally determined logK oc The value is 1.84, and the predicted and experimental data are in good agreement.
Embodiment 3
[0031] Given a compound 2-chlorodiphenyl ether, to predict its logK oc value. First, according to the structural information of 2-chlorodiphenyl ether, use the Gaussian09 software package to optimize its structure. Based on the Gaussian optimized structure, use Draogon6.0 software to calculate nHM, WiA_Dt, H_D / Dt, HATS4v, R3e+, nRCN, nR =CRX, O-061, P-117, CATS2D_05_NL, B03N-S, B08Br-Br, F02S-S, F05N-O and MLOGP2 have values of 1, 6.462, 192.8, 0.24, 0.111, 0, 0, 0, 0, 0, 0, 0, 0, 0 and 19.499. The h value calculated according to the formula (2) is 0.007 (oc The predicted value of 3.34, its experimentally determined logK oc The value is 3.47, and the data of the predicted value and the experimental value are in good agreement.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com