Telaprevir intermediate in B crystal form and synthesis method thereof
A technology of telaprevir and a synthesis method, applied in the field of drug synthesis, can solve problems such as low purity, influence on drug efficacy, etc., and achieve the effects of good appearance, good temperature and humidity stability, and short use time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
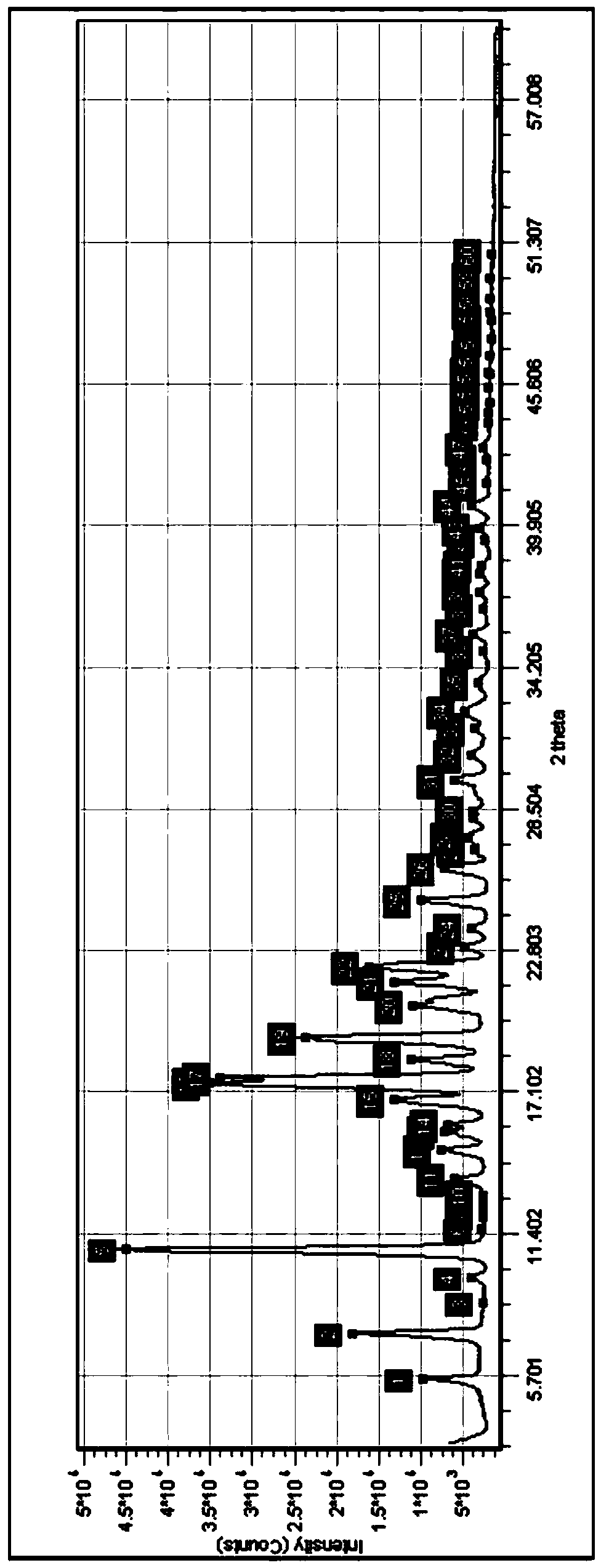
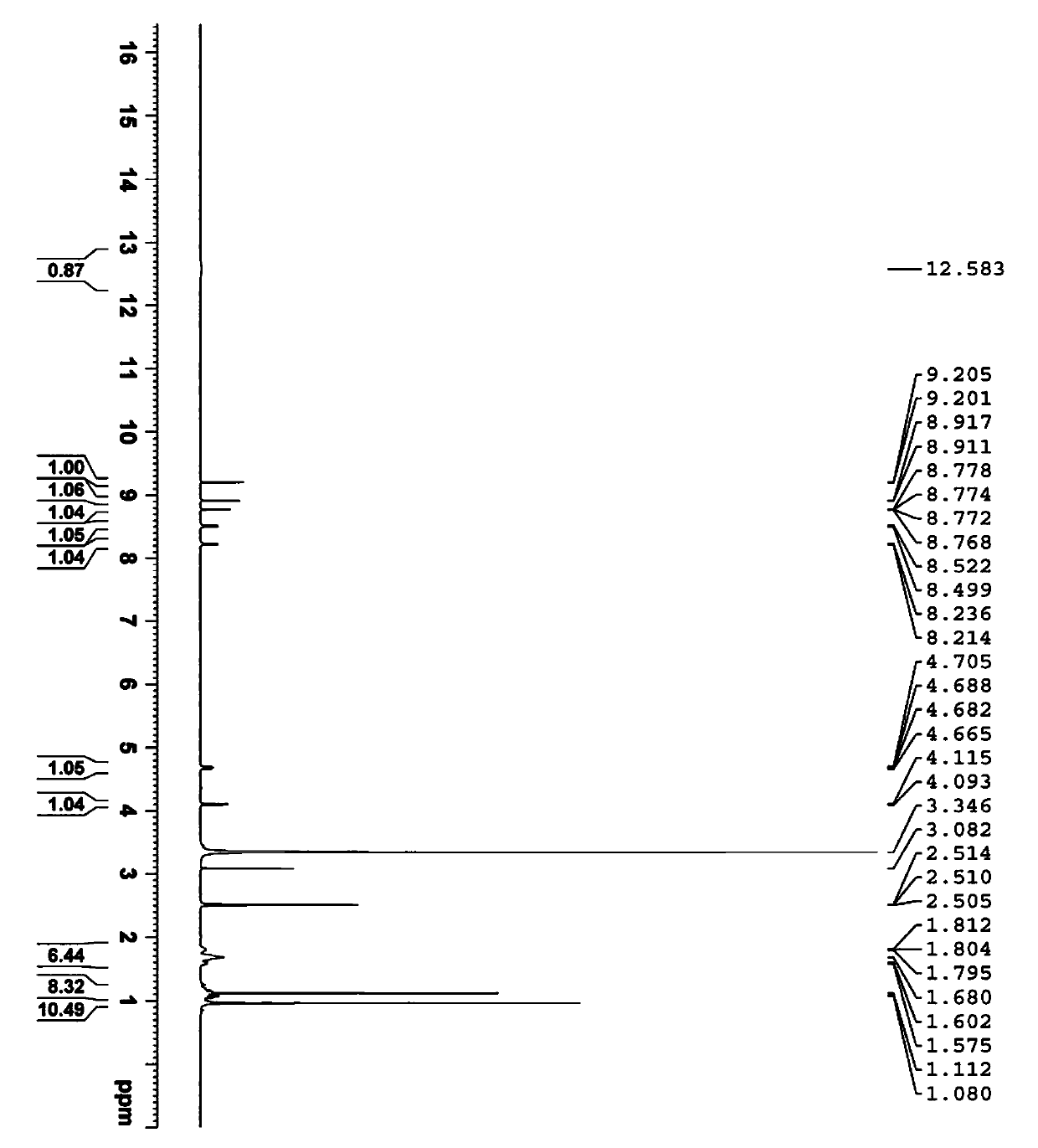
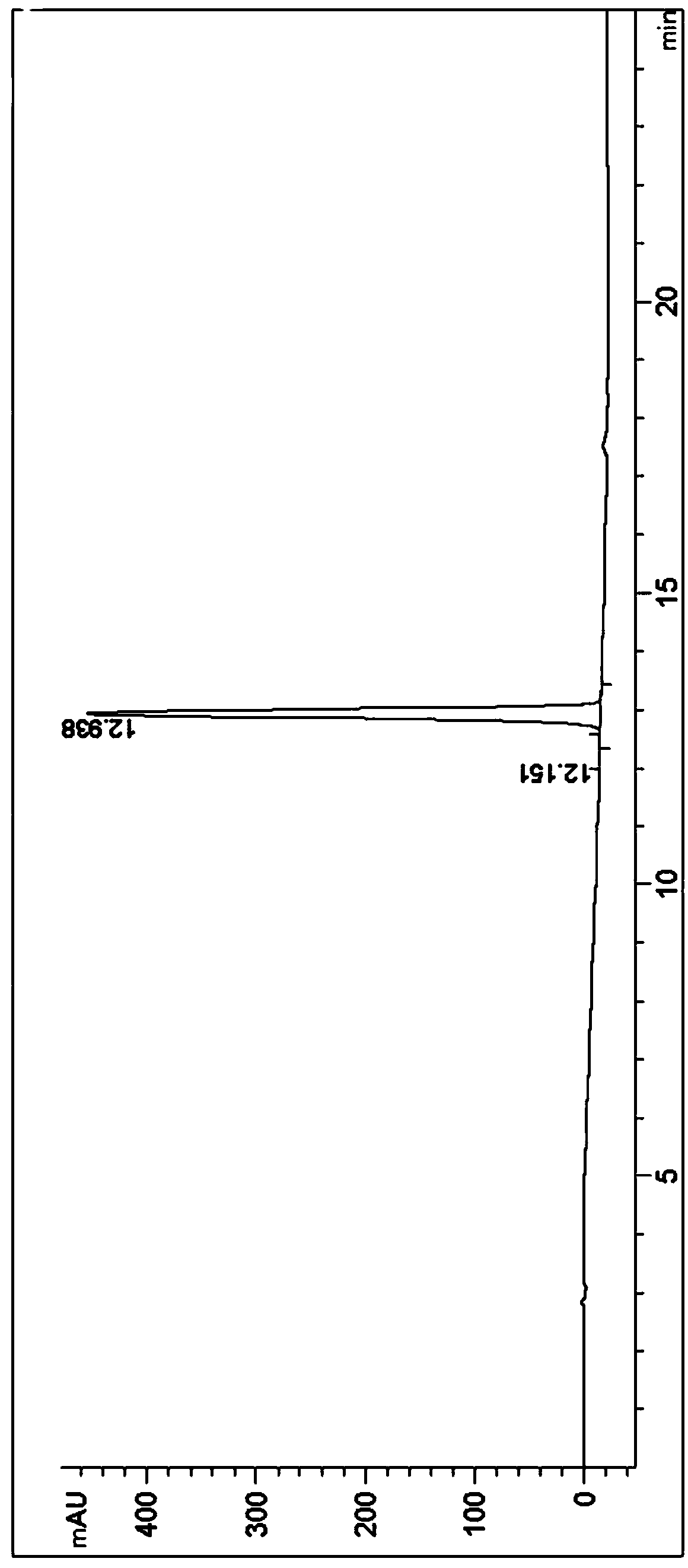
[0051] 10g telaprevir intermediate (2S)-2-cyclohexyl-N-(2-pyrazinylcarbonyl) glycyl-3-methyl-L-valine was added to 25mL absolute ethanol, in Heat to reflux at 80°C until the solution is clear, concentrate to dryness at 40°C, then add 50mL of ethyl acetate to make slurry for 4 hours, let stand, collect crystals by suction filtration, and dry to obtain 9.3g telaprevir intermediate Form B (the crystal lattice contains a small amount of ethyl acetate solvent). The purity is 99.10%, and the chiral purity is 100%.
Embodiment 2
[0053] Under stirring, add 30mL absolute ethanol to 10g telaprevir intermediate (2S)-2-cyclohexyl-N-(2-pyrazinylcarbonyl)glycyl-3-methyl-L-valine , heated at 80°C until the telaprevir intermediate was completely dissolved to form a reaction liquid, and when the reaction liquid was cooled to 30°C, n-heptane was added until all the solids were precipitated, stirred for 4 hours and left to stand, the crystals were collected by suction filtration, and dried , to obtain 8.7 g telaprevir intermediate B crystal form. The purity is 99.36%, and the chiral purity is 99.86%.
Embodiment 3
[0055] Under stirring, 40mL dichloromethane was added to 5g telaprevir intermediate (2S)-2-cyclohexyl-N-(2-pyrazinylcarbonyl)glycyl-3-methyl-L-valine , heated and refluxed at 40° C. for 3 hours, cooled naturally to room temperature, collected crystals by suction filtration, and dried to obtain 4.2 g of telaprevir intermediate B crystal form. The purity is 99.30%, and the chiral purity is 99.62%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Injection volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com