Copper complex having electrocatalytic activity on hydrogen peroxide and preparation method thereof
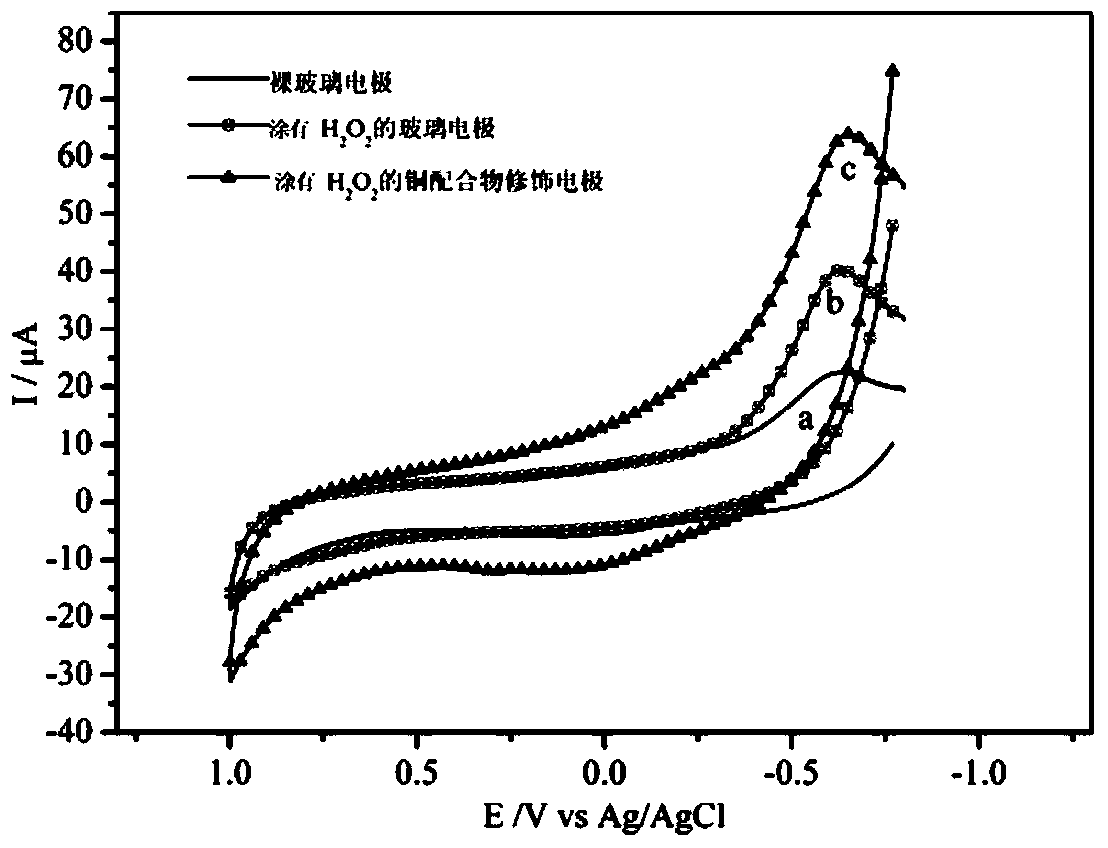
A technology of copper complexes and organic ligands, applied in the field of material chemistry, can solve the problems of poor stability of semiconductor oxides, low catalytic activity, complex catalyst preparation process, etc., and achieve good electrocatalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
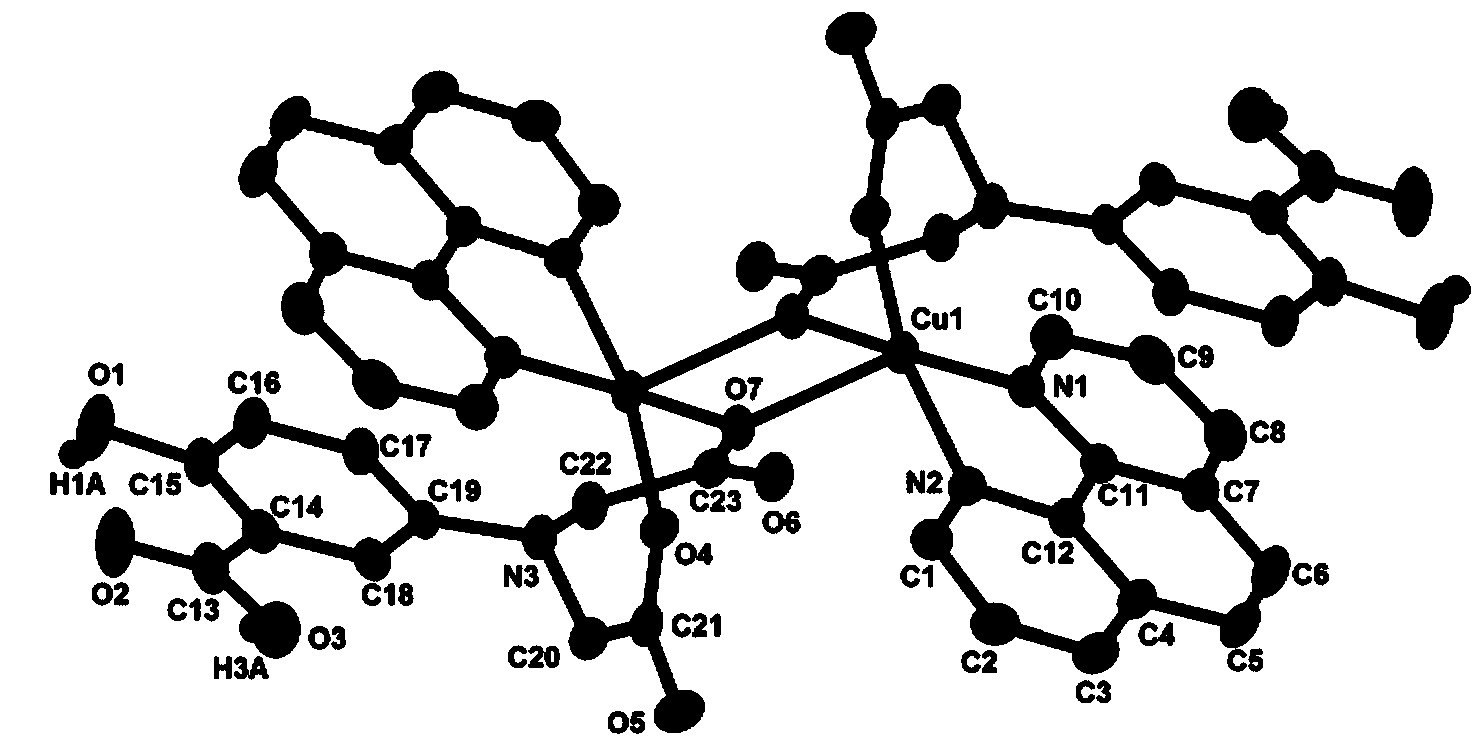
[0020] Weigh CuSO 4 ·5H 2 O (2.497g, 10mmol), H 4 Add L (0.269g, 1.0mmol) and 1,10-phenanthroline (0.180g, 1.0mmol) to 5.0mL distilled water, stir to dissolve; seal the reaction device and heat at 60°C for 60min, then take it out and cool to Room temperature; then use dilute KOH solution to adjust the mixed solution to be weakly acidic (pH=5); let it stand at room temperature, and slowly evaporate the solvent until crystals are precipitated. The solution was removed, and the massive crystals obtained by natural air drying were copper complexes.
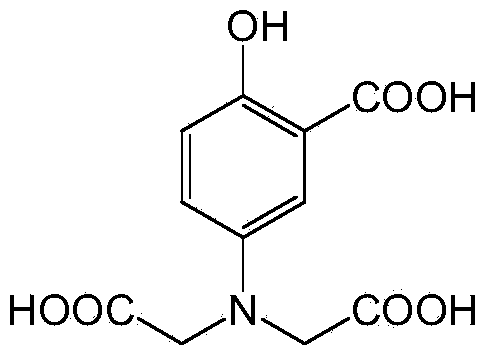
[0021] Wherein, the ligand 5-(bis(carboxymethyl)amino)-2-hydroxybenzoic acid (H 4 L) Preparation method: KOH solution (12M, 20mL) was added dropwise into sodium chloroacetate (13.978g, 0.12mol) aqueous solution (20mL) and stirred, then 5-aminosalicylic acid (4.59g, 0.03mmol) Slowly added to the above mixture, reacted at 85°C for 30h. After the reaction, cool to room temperature, acidify with HCl solution (6mol / L) until precipitat...
Embodiment 2
[0023] Weigh CuCl 2 2H 2 O (0.170g, 1.0mmol), H 4 Add L (2.692g, 10mmol) and 1,10-phenanthroline (1.802g, 10mmol) to 40mL of distilled water, stir to dissolve; seal the reaction device and heat at 90°C for 15min, then take it out and cool to room temperature; Use dilute KOH solution to adjust the mixed solution to be weakly acidic (pH=7); let it stand at room temperature, and slowly evaporate the solvent until crystals are precipitated. The solution was removed, and the massive crystals obtained by natural air drying were copper complexes.
Embodiment 3
[0025] Weigh Cu(NO 3 ) 2 ·3H 2 O (0.483g, 2.0mmol), H 4 Add L (2.692g, 10mmol) and 1,10-phenanthroline (1.802g, 10mmol) to 15mL of distilled water, stir to dissolve; seal the reaction device and heat at 80°C for 30min, then take it out and cool to room temperature; Use dilute KOH solution to adjust the mixed solution to be weakly acidic (pH=6); let it stand at room temperature, and slowly evaporate the solvent until crystals are precipitated. The solution was removed, and the massive crystals obtained by natural air drying were copper complexes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com