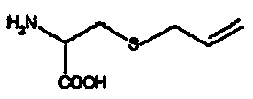
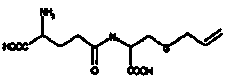
Preparation method of sulfur-allyl-L-cysteine
A technology of cysteine and allyl group, applied in the field of bioengineering, can solve the problems of complex production process and low yield, and achieve the effect of simple preparation process, high production efficiency and short time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of sulfur-allyl-L-cysteine, comprising the steps of:
[0033] Take 60.6g gamma -glutamyl-thio-allyl- L - Cysteine, dissolved in 10 L of NH with a concentration of 0.05 mol / L and a pH of 9.5 3 -NH 4 Add 0.1L of γ-glutamyl transpeptidase solution (specific activity 500U / mL) to the Cl buffer solution, stir and react at 37°C for 60 minutes, and measure the sulfur-allyl-L-cysteine in the solution after the reaction The concentration was 2.74g / L, and the yield of sulfur-allyl-L-cysteine was 85.1%.
Embodiment 2
[0035] A preparation method of sulfur-allyl-L-cysteine, comprising the steps of:
[0036] The purified γ-glutamyl transpeptidase was embedded in chitosan and cross-linked with glutaraldehyde to obtain immobilized γ-glutamyl transpeptidase, and its activity was determined to be 53U / g; 606g gamma -glutamyl-thio-allyl- L -Cysteine is dissolved in 100L of NH with a concentration of 0.05mol / L and a pH of 10.0 3 -NH 4 In Cl buffer solution, add 500 g of immobilized γ-glutamyl transpeptidase to the buffer solution, stir and react at 37°C for 60 minutes to obtain a reaction solution; filter and recover the immobilized γ-glutamyl transpeptidase in the reaction solution , the concentration of sulfur-allyl-L-cysteine in the remaining reaction solution was detected to be 2.64g / L, and the yield of sulfur-allyl-L-cysteine was 82.0%.
Embodiment 3
[0038] A preparation method of sulfur-allyl-L-cysteine, comprising the steps of:
[0039] The purified γ-glutamyl transpeptidase was embedded in alginate and cross-linked with anhydrous butylene dioic acid-ethylene to obtain immobilized γ-glutamyl transpeptidase, and its activity was determined to be 85 U / mg; Take 1212g gamma -glutamyl-thio-allyl- L - Cysteine dissolved in 100L of NH with a concentration of 0.05mol / L and a pH of 9.0 3 -NH 4 In Cl buffer solution, stir and react at 37°C for 120 minutes to obtain a reaction solution; filter and recover the immobilized γ-glutamyl transpeptidase in the reaction solution, and detect sulfur-allyl-L-cysteine in the remaining reaction solution The acid concentration was 5.16g / L, and the yield of sulfur-allyl-L-cysteine was 80.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com