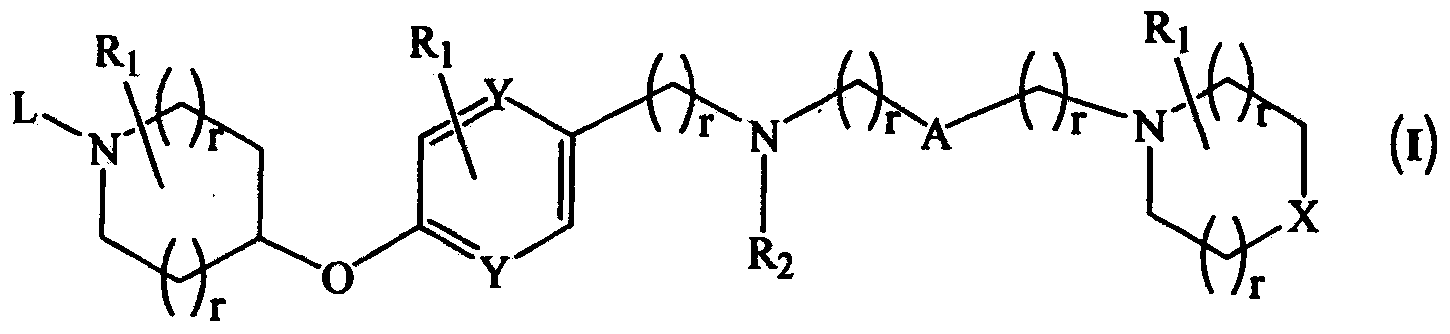
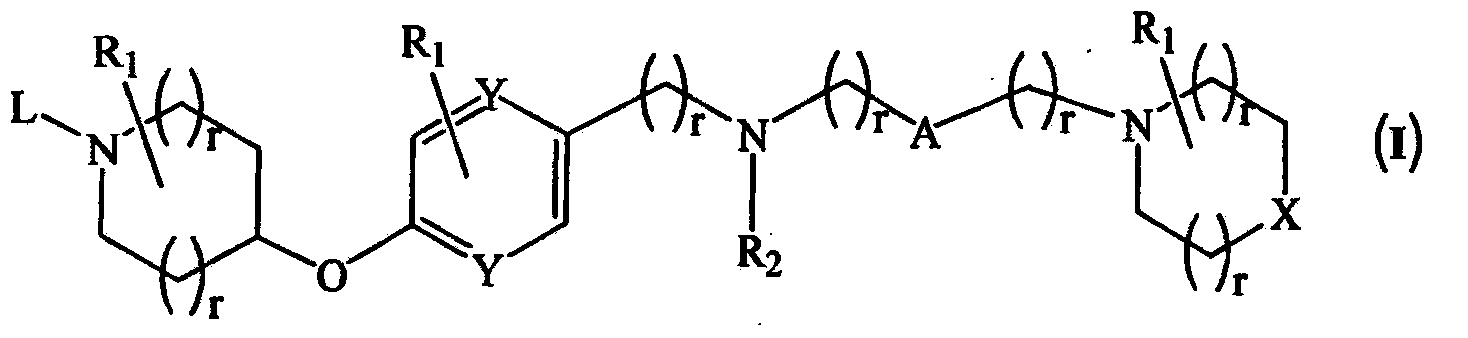
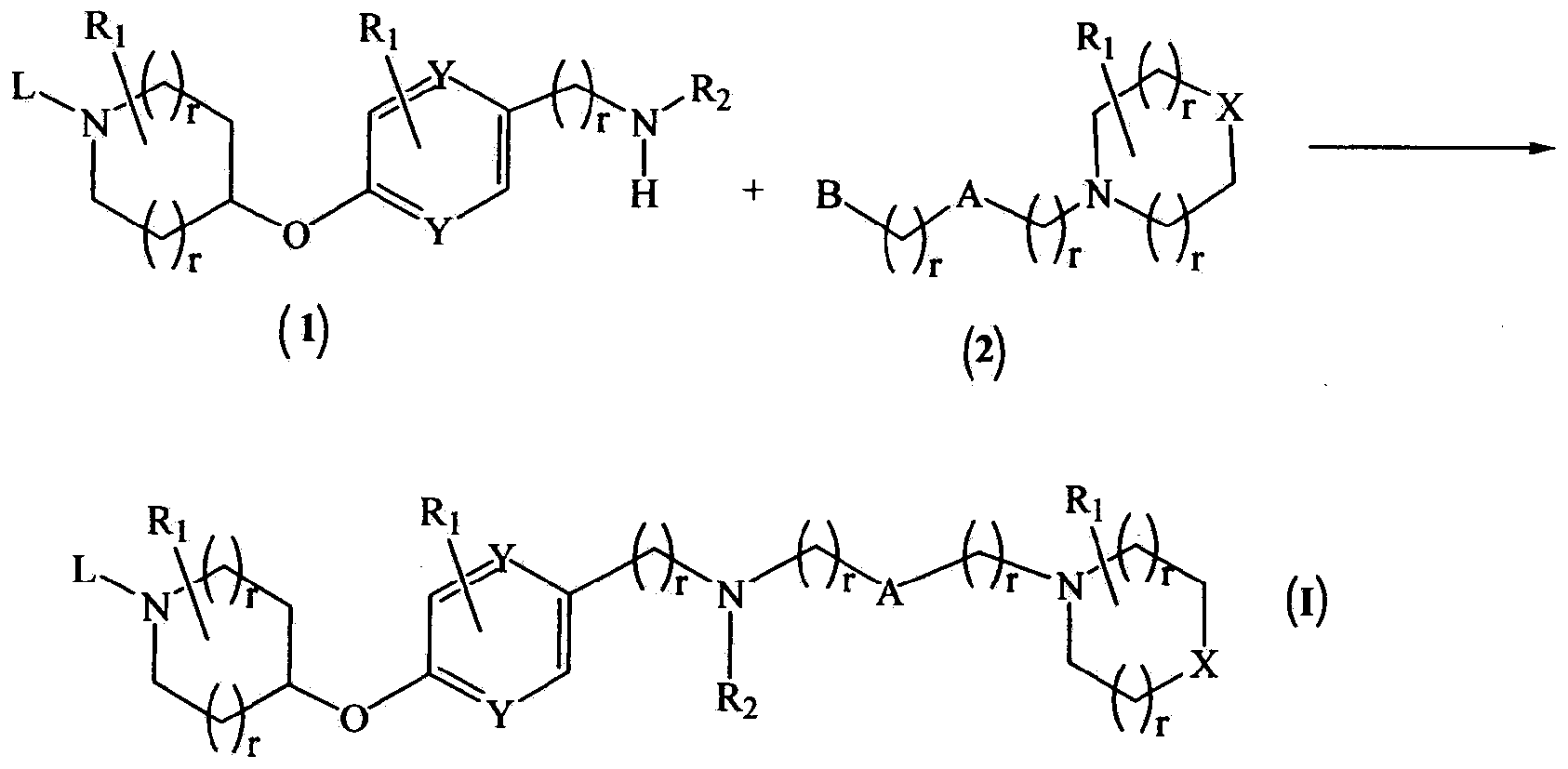
Novel compounds as histamine H3 receptor ligands
A compound, the technology of acetamide, applied in the field of new compounds as histamine H3 receptor ligands, can solve the problem of not launching compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Example 1: Preparation of N-[4-(1-cyclobutylpiperidin-4-yloxy)phenyl]-2-(morpholin-4-yl)-acetamide dihydrochloride
[0160] Step (i): Preparation of 2-chloro-N-[4-(1-cyclobutylpiperidin-4-yloxy)phenyl]acetamide
[0161] To a solution of 4-[(1-cyclobutyl-4-piperidinyl)oxy]aniline (81 g, 0.329 mol, obtained in preparation 1) in dichloromethane (1L) under a nitrogen atmosphere at 0°C Add triethylamine (66.5g, 0.658 mol). The resulting material was then treated dropwise with a solution of chloroacetyl chloride (44.6 g, 0.395 mol) in dichloromethane (1 L) at 0°C and stirred at 0°C for 1 hour. The reaction mixture was washed with water, dried over sodium sulfate and concentrated under vacuum, and the crude compound thus obtained was purified by flash chromatography (methanol:chloroform, 2:8) to obtain 76.1 g of the title compound (yield: 72%).
[0162] 1 H-NMR (δppm): 1.55-1.99 (12H, m), 2.49-2.67 (3H, m), 4.19 (2H, s), 4.26-4.28 (1H, m), 6.88-6.90 (2H, d, J =8.9Hz), 7.44-7.46 (2...
Embodiment 2
[0173] HPLC: 99.54%; MP: 249.2-251.5°C; salt content: 16.09% (dihydrochloride); Example 2: 2-[4-(1-cyclobutylpiperidin-4-yloxy)benzene Preparation of 1-(morpholin-4-yl)ethanone hydrochloride
[0174] Step (i): Preparation of 2-[4-(1-cyclobutylpiperidin-4-yloxy)phenylamino]-1-(morpholin-4-yl)ethanone
[0175] Stir 4-(1-cyclobutylpiperidin-4-yloxy)aniline (0.5g, 0.002mol), 2-chloro-1-(morpholin-4-yl)ethanone (0.5g) at reflux temperature , 0.003) and potassium carbonate (0.56g, 0.004mol) in dimethylformamide (25ml). After the reaction was completed, the mixture was concentrated under reduced pressure and the residue was partitioned between ethyl acetate (250 mL) and water (250 mL). The combined organic layer was washed with brine solution, dried over sodium sulfate and concentrated under reduced pressure. The crude compound was purified by flash chromatography (chloroform:triethylamine, 9.5:0.5) to obtain 0.3 g of the title compound (yield: 40%).
[0176] Step (ii): Preparation of 2...
Embodiment 3
[0180] Example 3: Preparation of N-[4-(1-cyclobutylpiperidin-4-yloxy)-2-fluorophenyl]-2-(morpholin-4-yl)acetamide dihydrochloride
[0181] Step (i): Preparation of tert-butyl 4-[3-fluoro-4-(2-(morpholin-4-yl)acetylamino)phenoxy]piperidine-1-carboxylate
[0182] At reflux temperature 4-[4-(2-chloroacetylamino)-3-fluorophenoxy]piperidine-1-carboxylic acid tert-butyl ester (3.31g, 0.0085mol, obtained in Preparation 3), A mixture of morpholine (0.89 g, 0.01 mol) and potassium carbonate (1.75 g, 0.012 mol) in acetonitrile (30 mL) was stirred for 5 hours. The mixture was concentrated under reduced pressure, and the residue thus obtained was partitioned between ethyl acetate (50 mL) and water (50 mL). The resulting aqueous phase was extracted with ethyl acetate (2×50 mL). The combined organic layer was washed with brine solution, dried over sodium sulfate and concentrated. The crude compound was purified by flash chromatography (ethyl acetate:hexane, 3:7) to obtain 3.1 g of the title ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com