Temperature fluorescent probe based on triaryl boron compound and its preparation and application
A compound, temperature-sensitive technology, applied in the direction of thermometers, thermometers, and compounds containing elements of Group 3/13 of the periodic table with physical/chemical changes, which can solve the problem of difficult to achieve in-situ detection in micro-areas and susceptible to environmental interference. , narrow application temperature and other problems, to achieve the effect of no correction, wide practical range, wide temperature measurement range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Preparation of compound Ia, its reaction scheme is as follows:
[0069]
[0070] Slowly add 0.8mL (2.22M) n-butyl lithium dropwise into 20ml ether solution containing 480mg 1-bromopyrene at -78°C under the protection of nitrogen. Stirring was continued for 1 hour. Then cool down to-78°C again, slowly add 5ml of ether solution containing 240mg 2,4,6-triisopropylphenylboronic acid dimethyl ester dropwise to the above solution, continue to stir for 30 minutes after the dropwise addition is completed, and then return to to room temperature and stirred overnight. After the reaction was completed, it was washed with water and separated on a silica gel column (developing solvent: petroleum ether: dichloromethane = 5:1, volume ratio) to obtain 380 mg of the product with a yield of 72%. Compound Ia: 1 HNMR (400MHz, CDCl 3 )δ=8.23(d,J=7.74Hz,2H),8.20-8.15(m,6H),8.12(d,J=5.4Hz,2H),8.02(d,J=9.22Hz,2H),7.97( d,J=8.82Hz,4H),7.57(d,J=9.22Hz,2H),7.08(s,2H),3.03-2.96(m,1H),2.93-...
Embodiment 2
[0073] The intermediate Ih of compound Id is prepared, and its molecular structural formula is:
[0074]
[0075] Its reaction scheme is as follows:
[0076]
[0077] 100mg Tris(dibenzylideneacetone)dipalladium(0)chloroform adduct, 1.5g sodium tert-butoxide, 120mg bis-diphenylphosphonobinaphthyl
[0078] (2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl) and 1.80g of 1,6-dibromopyrene were added to 50ml of degassed toluene, stirred for 15 minutes under nitrogen protection, and then added 0.5ml tetrahydropyrrole, then slowly warming up to 90 ° C, continue to stir for 3 hours. After the reaction was completed, remove incompatible substances by filtration, wash with water three times, and separate on a silica gel column (petroleum ether: dichloromethane = 1:1, volume ratio) to obtain 910 mg of the product with a yield of 52%. Intermediate compound Ih: 1 H NMR (400MHz, CDCl 3 )δ=8.41(d,J=9.1Hz,1H),8.19(d,J=9.1Hz,1H),8.14(d,J=8.2Hz,1H),8.07(d,J=8.4Hz,1H) ,8.02(d,J=9.1Hz,1H),7...
Embodiment 3
[0080] Preparation compound Id, its reaction scheme is as follows:
[0081]
[0082] Compound Id was synthesized by the same method as that of Ia, and the yield was 66%. 1 H NMR (400MHz, CDCl 3 )δ=8.50(d,J=9.1Hz,2H),8.14(d,J=6.8Hz,2H),8.00(t,J=8.0Hz,4H),7.85(q,J=8.3Hz,4H) ,7.56(s,2H),7.43(d,J=8.5Hz,2H),7.06(s,2H),3.61(s,8H),2.98(t,J=6.7Hz 1H),2.91(t,J =5.7Hz,2H),2.11(s,8H),1.35(d,J=6.8Hz,6H),0.94(d,J=5.9Hz,6H),0.78(d,J=6.0Hz,6H); MALDI-TOF:M + 754.5; Elemental Analysis (C 55 h 55 BN 2 ): C 87.51%, H 7.34%, N 3.71%; measured: C 87.10%, H 7.26%, N 3.57%.
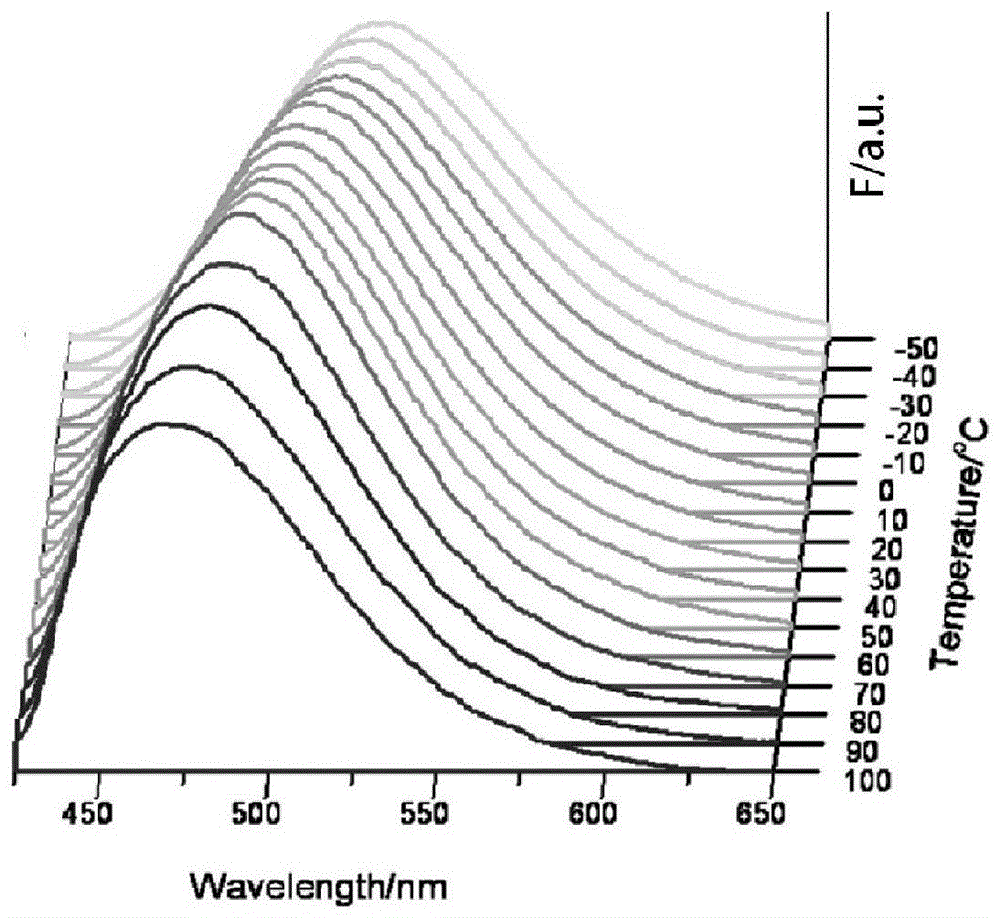
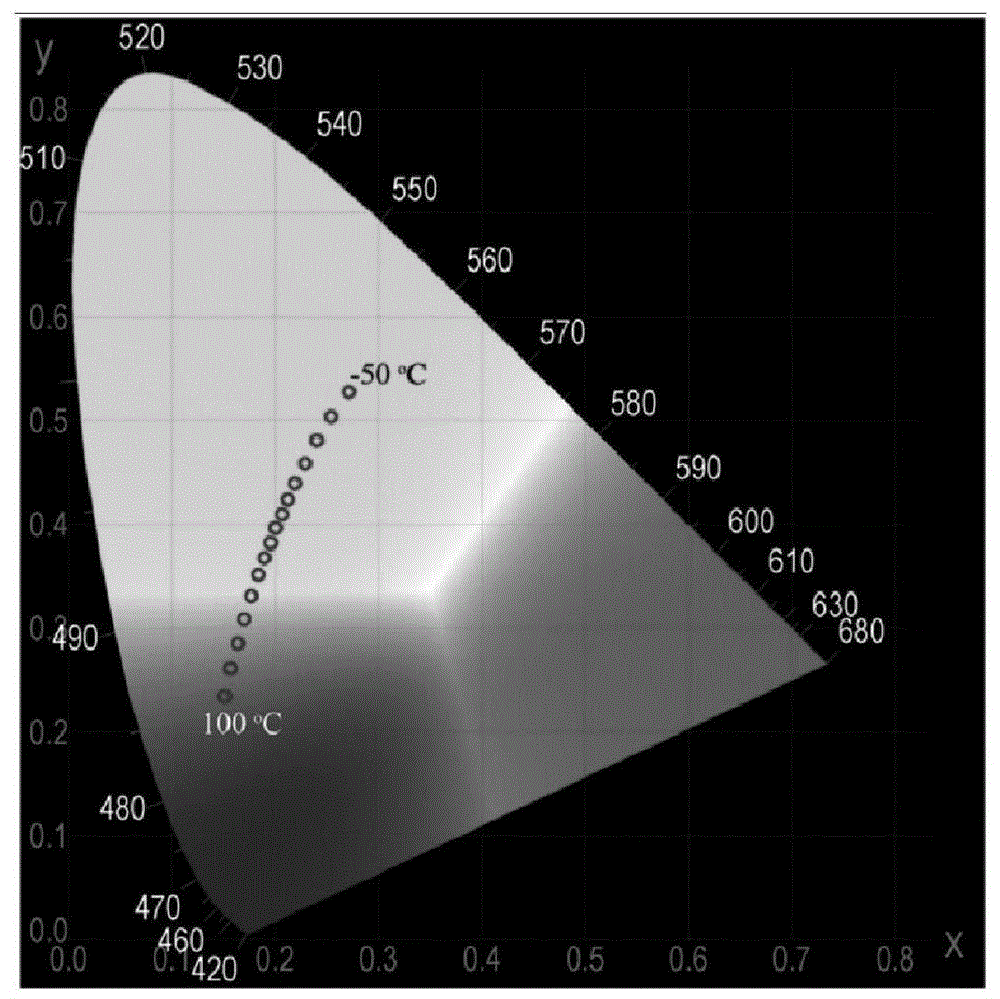
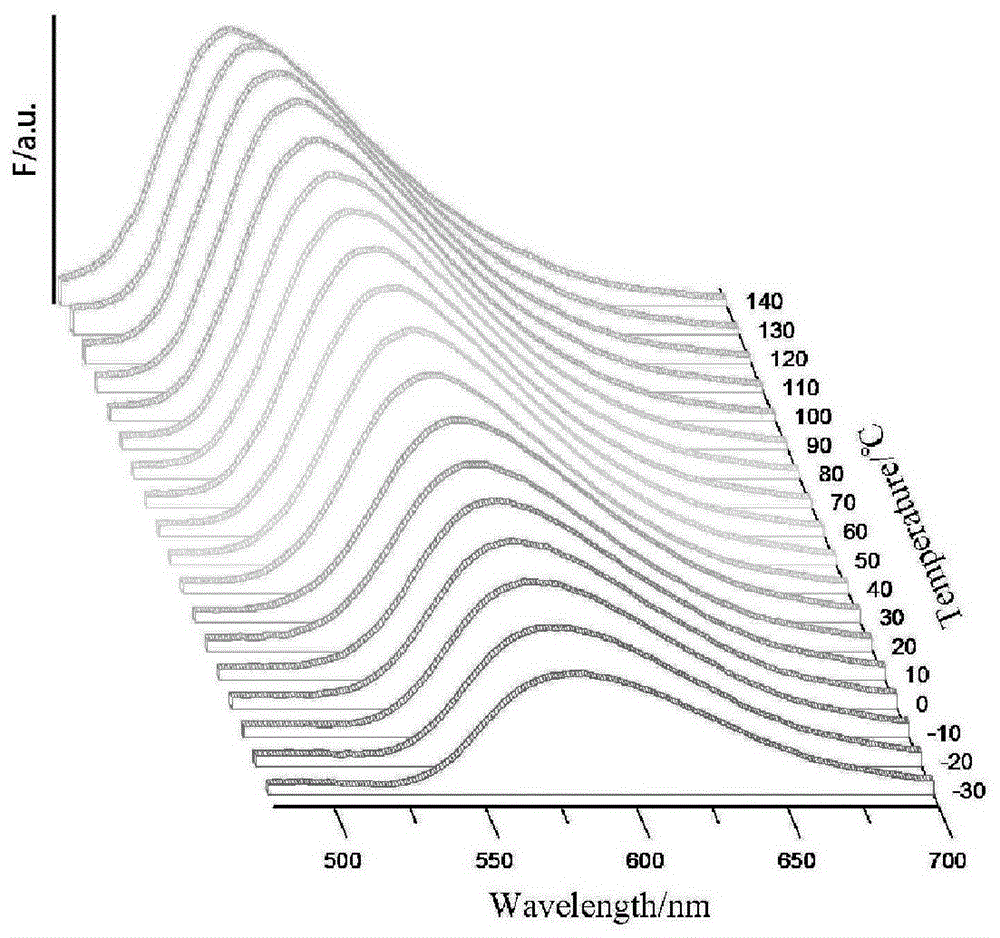
[0083] image 3 is the fluorescence spectrum of compound Id at different temperatures (350nm excitation), Figure 4 It is a chromaticity diagram converted from the spectrum diagram of compound Id. As the temperature increases, the luminescence of the compound gradually changes from orange to green.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com