Method for synthesizing p-toluenesulfonic acid-catalyzed dimethyl malonate
A technology of dimethyl malonate and p-toluenesulfonic acid, which is applied in the field of malonate preparation, can solve the problems of easy decomposition of cyanoacetic acid and consumption of sulfuric acid acid waste water, etc., so as to reduce the amount of use, reduce the amount of use and the amount of waste water generated , avoid the effect of high salt content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
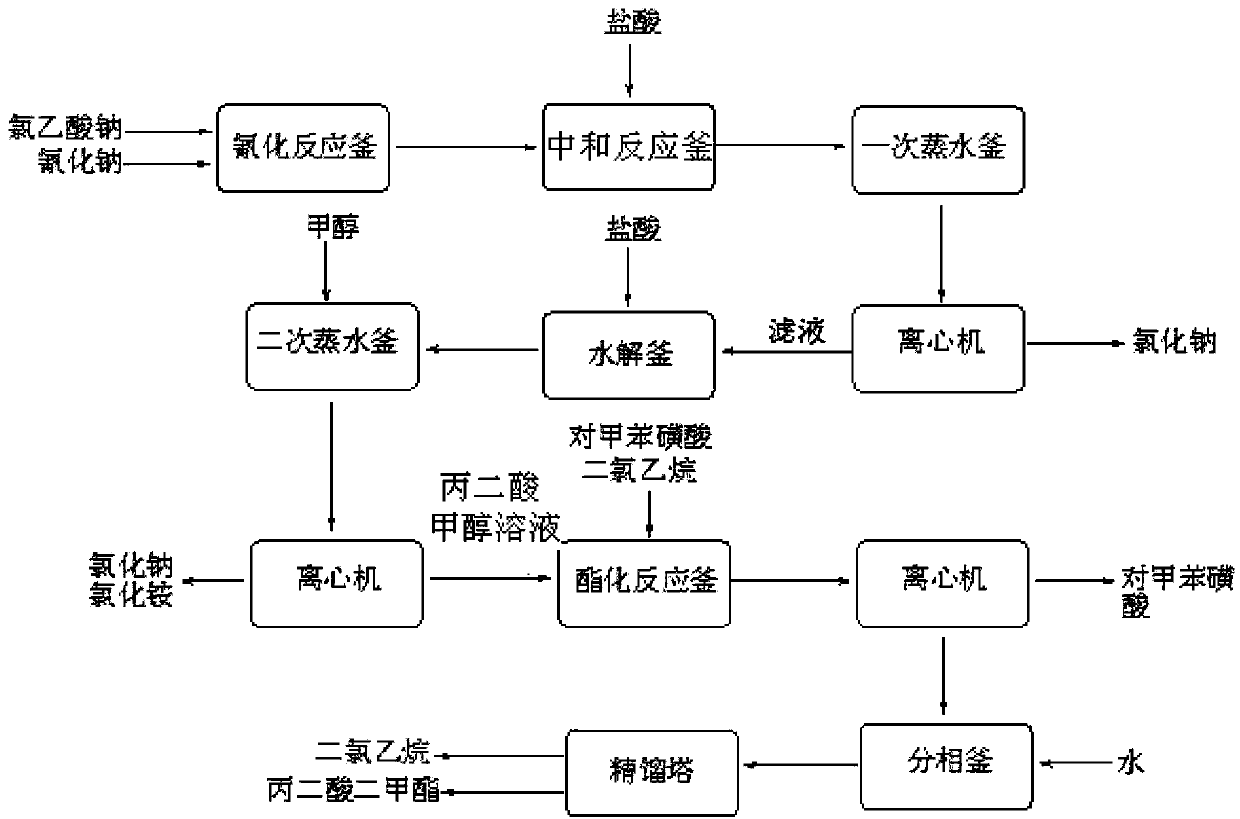
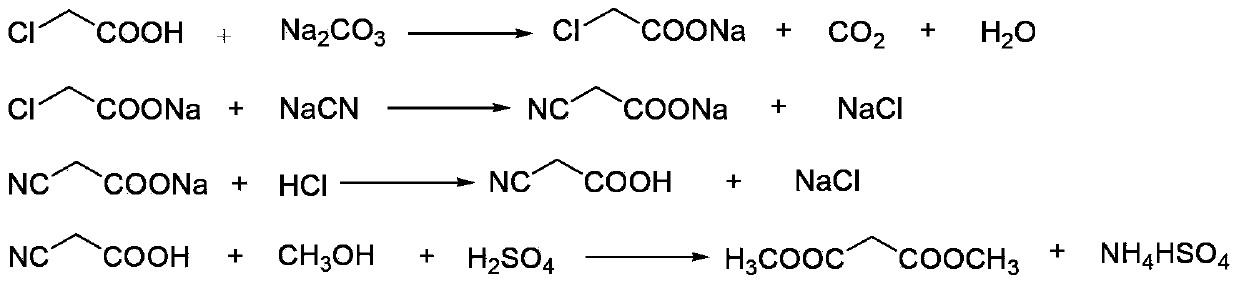
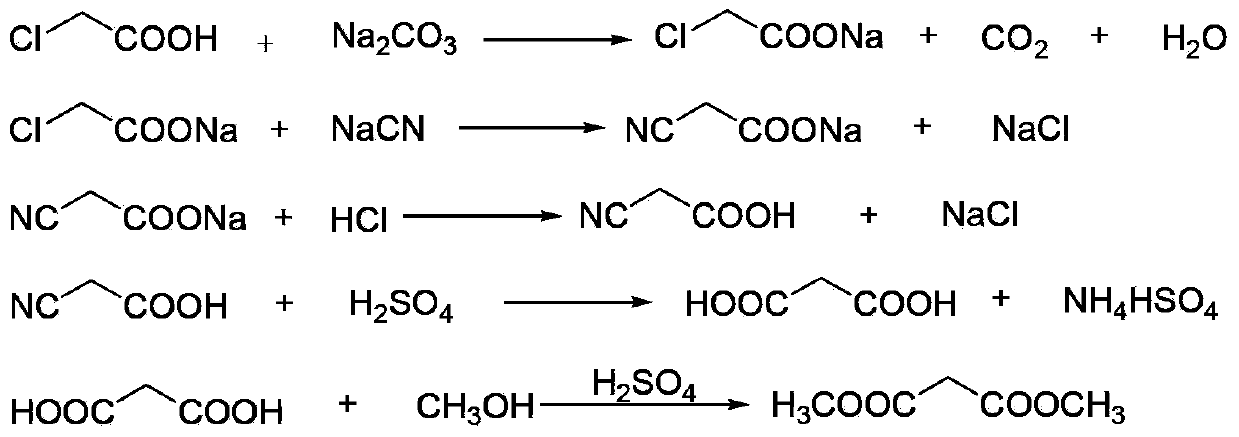
[0034] (1) Neutralization reaction: add 900kg of chloroacetic acid (content 98%, 9.30kmol) to 620kg of water, stir and dissolve, neutralize with aqueous sodium carbonate to pH=6.5-7.0, and generate sodium chloroacetate;
[0035] (2) Cyanide reaction: transfer the sodium chloroacetate obtained in step (1) into the cyanide reaction kettle, raise the temperature to 50°C, then add 1530kg (4.69kmol) of 30% sodium cyanide aqueous solution dropwise, when the reaction temperature reaches 85 After -90°C, stop heating and start to cool. When the reaction temperature reaches 105°C, the reaction ends and is cooled to room temperature to obtain an aqueous solution of sodium cyanoacetate;
[0036] (3) Acidification reaction: transfer the sodium cyanoacetate aqueous solution obtained in step (2) into the acidification reaction kettle, stir, and slowly add 1000L (content, 31%, 10.14kmol) hydrochloric acid into the acidification reaction kettle from the metering tank, after acidification to gen...
Embodiment 2
[0043] Embodiment 2 is directly applying the p-toluenesulfonic acid that embodiment 1 reclaims, and its preparation process is:
[0044] (1) Esterification reaction: Take the malonic acid methanol solution prepared in Example 1, add 550 L of dichloroethane and the p-toluenesulfonic acid recovered in Example 1 to the esterification reaction kettle, and control the temperature of the kettle to 65-70°C Perform an esterification reaction to generate dimethyl malonate, and the water generated by the reaction is continuously taken out of the system through azeotropy, and 200L of methanol is added after 2 hours;
[0045] (2) Extraction and phase separation: After the esterification is completed, cool to room temperature, centrifuge, recover 69kg of p-toluenesulfonic acid, add 150L of water, stir for 30 minutes, let stand for 40-60 minutes, then separate the phases, and wash the organic phase with saturated sodium carbonate solution Then transfer to rectification tower, rectification ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com