Semi-rigid double-imidazole organic-ligand-based transition metal compound containing N-S dicarboxylic acid, as well as synthetic method and application thereof
A technology of organic ligands and transition metals, applied in the field of transition metal complexes and their synthesis, can solve the limitation of the length of organic ligands, the flexibility of ligands, the number of coordination sites and the coordination ability, and the synthesis yield of transition metal functional complexes. There are only problems such as poor catalytic degradation effect of water-soluble organic pollutants, etc., to achieve the effect of good catalytic degradation effect, shortening the synthesis cycle, and increasing coordination adaptability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Synthesis of [Co 2 (L) 2 (2-CMSN) 2 (H 2 O)]·H 2 O, wherein, L is 1,4-two (imidazol-1-methylene) benzene, the structural formula is: , 2-CMSN is 2-carboxymethylenethionicotinate
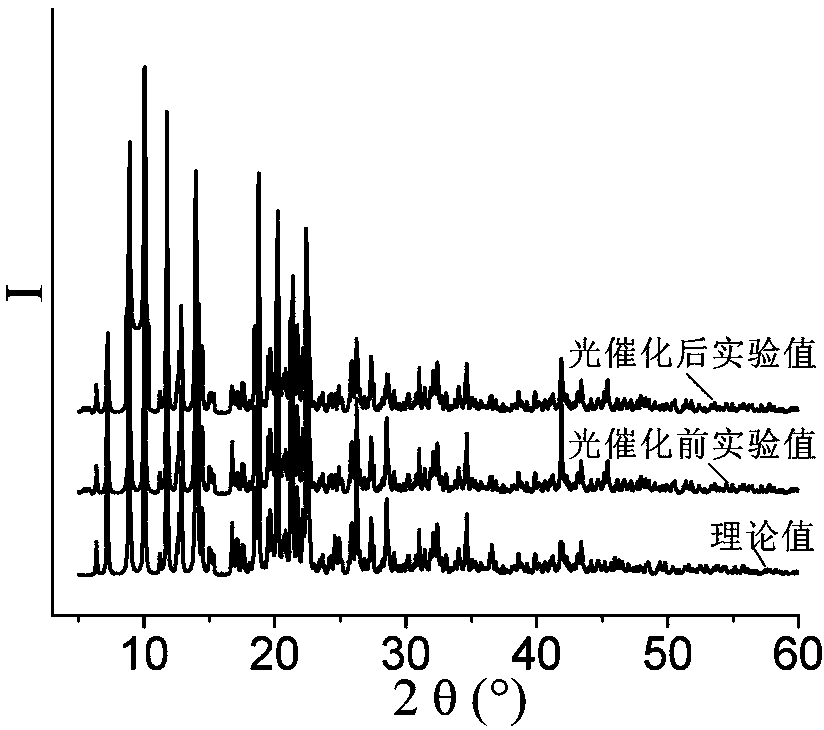
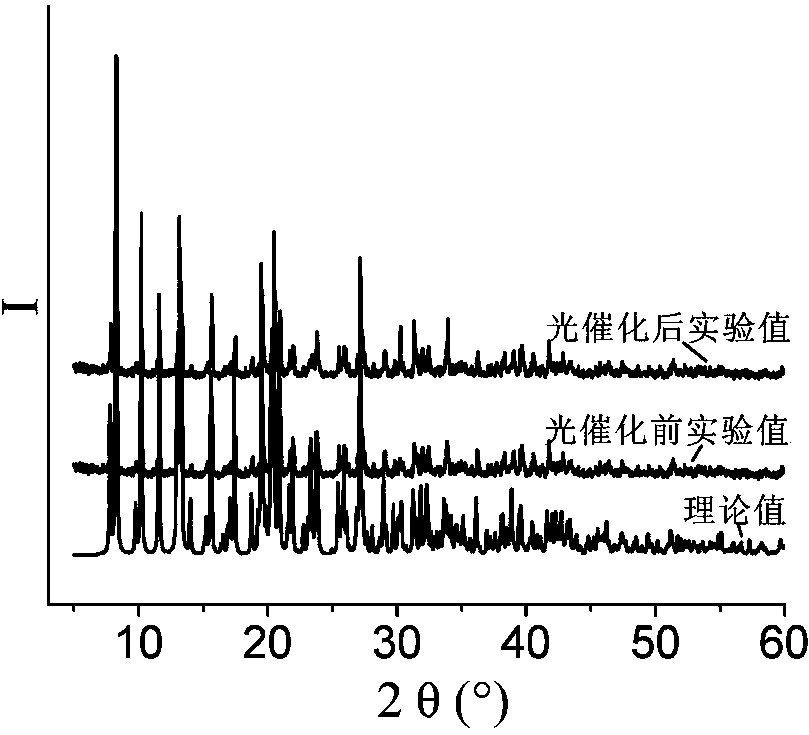
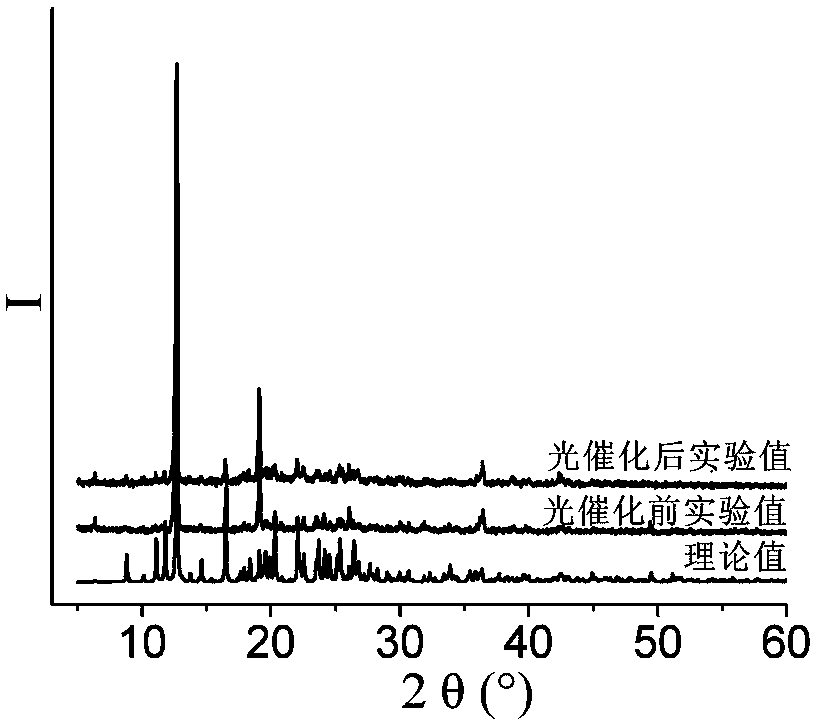
[0051] 0.1mmol Co(NO 3 ) 2 ·6H 2 O, 0.10mmol 1,4-bis(imidazol-1-methylene)benzene, 0.1mmol 2-carboxymethylenethionicotinic acid (the structural formula is: ) and 7.5mL H 2 Add O to a 50mL beaker in turn, stir at room temperature for 20min, adjust the pH to 6 with 0.1mol / L NaOH solution, pour it into a 25mL autoclave, heat up to 90°C at a heating rate of 15°C / h, and heat Under the condition of heat preservation for 48 h, the temperature was lowered to room temperature at a cooling rate of 5 °C / h to obtain pink blocky crystals, which were washed twice with deionized water and ethanol alternately, and dried naturally at room temperature to obtain [Co 2 (L) 2 (2-CMSN) 2 (H 2 O)]·H 2 O, the yield is 62%, and its coordination environment diagram is as follows Figure 7 As ...
Embodiment 2
[0052] Example 2 Synthesis of [Co 2 (L) 2 (2-CMSN) 2 (H 2 O)]·H 2 O, where L is 1,4-bis(imidazol-1-methylene)benzene, and 2-CMSN is 2-carboxymethylenethionicotinate
[0053] 0.2mmol Co(NO 3 ) 2 ·6H 2 O, 0.10mmol 1,4-bis(imidazol-1-methylene)benzene, 0.14mmol 2-carboxymethylenethionicotinic acid and 12mL H 2 Add O to a 50mL beaker in turn, stir at room temperature for 30min, adjust the pH to 6.1 with 0.5mol / L NaOH solution, pour it into a 25mL autoclave, heat up to 100°C at a heating rate of 10°C / h, and heat Under the condition of heat preservation for 48h, the temperature was lowered to room temperature at a cooling rate of 2.5 °C / h to obtain pink blocky crystals, washed alternately with deionized water and ethanol for 5 times, and dried naturally at room temperature to obtain [Co 2 (L) 2 (2-CMSN) 2 (H 2 O)]·H 2 O, the yield is 84%, and its coordination environment diagram is as follows Figure 7 As shown, its two-dimensional network diagram is shown as Figure 8...
Embodiment 3
[0054] Example 3 Synthesis of [Co 2 (L) 2 (2-CMSN) 2 (H 2 O)]·H 2 O, where L is 1,4-bis(imidazol-1-methylene)benzene, and 2-CMSN is 2-carboxymethylenethionicotinate
[0055] 0.3mmol Co(NO 3 ) 2 ·6H 2 O, 0.10mmol 1,4-bis(imidazol-1-methylene)benzene, 0.2mmol 2-carboxymethylenethionicotinic acid and 14mL H 2 Add O to a 50mL beaker in turn, stir at room temperature for 60min, adjust the pH to 7.0 with 1mol / L NaOH solution, pour it into a 25mL autoclave, and raise the temperature to 105°C at a heating rate of 5°C / h. Keep it warm for 60 hours, lower the temperature to room temperature at a cooling rate of 8°C / h, and obtain pink blocky crystals, wash them alternately with deionized water and ethanol for 3 times, and dry naturally at room temperature to obtain [Co 2 (L) 2 (2-CMSN) 2 (H 2 O)]·H 2 O, the yield is 74%, and its coordination environment diagram is as follows Figure 7 As shown, its two-dimensional network diagram is shown as Figure 8 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com