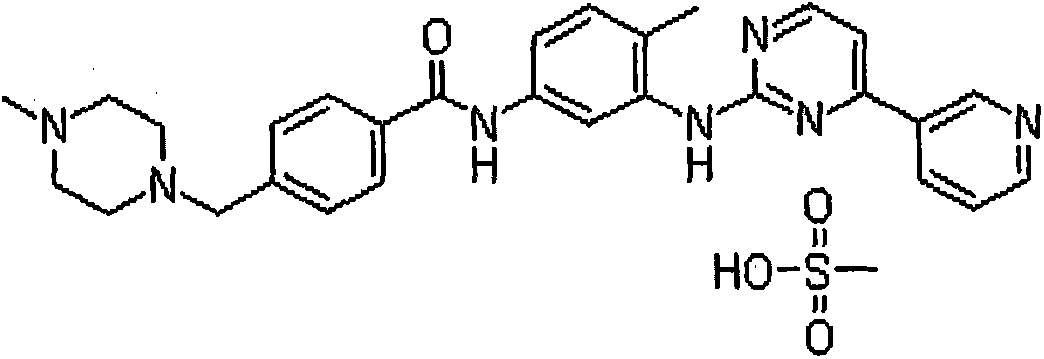
Imatinib mesylate pill and preparation method thereof
A technology of imatinib mesylate and imatinib mesylate, which is applied in the field of imatinib mesylate pills and its preparation, and can solve problems such as inconvenience for patients to take, large size, and difficulty in tablet disintegration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
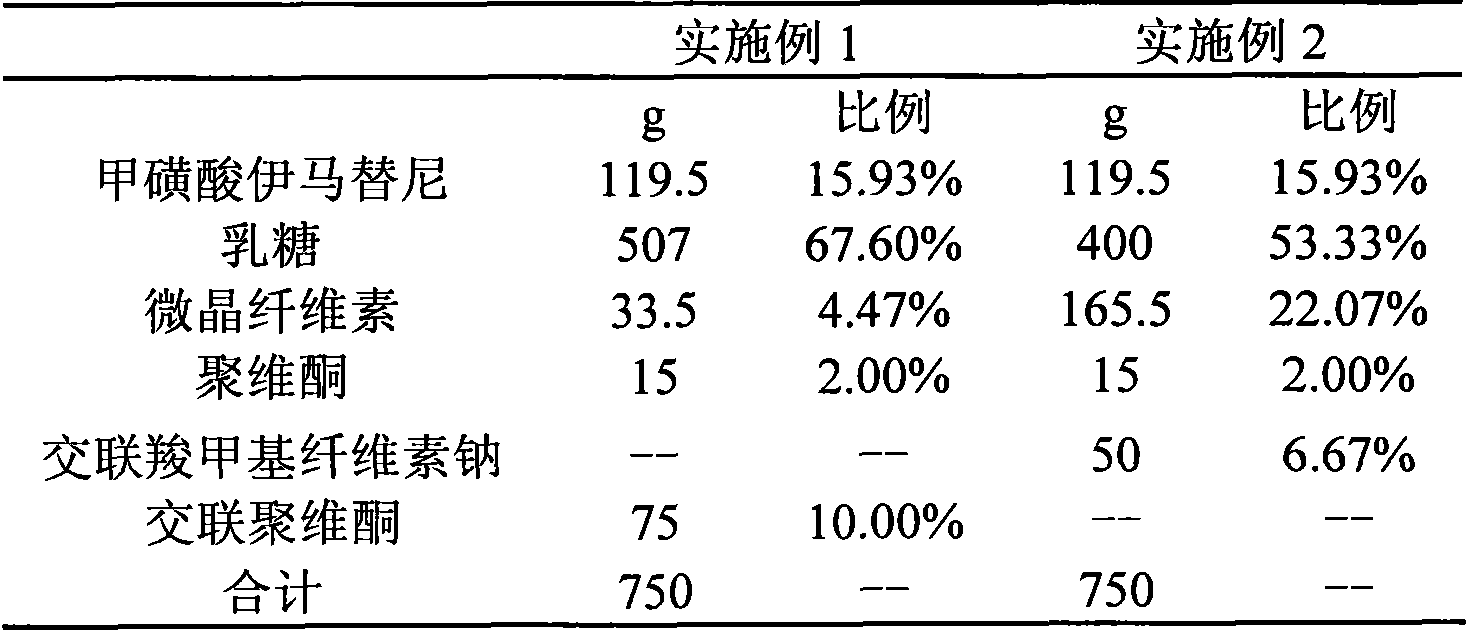
Embodiment 1-2
[0037]
[0038] 1. Preparation process:
[0039] 2. Mix imatinib mesylate, lactose, crospovidone or croscarmellose sodium in a wet granulation pot,
[0040] 3. Dissolve and disperse povidone into 95% ethanol solution, add it to the granulation pot to prepare soft material,
[0041] 4. Extrude the soft material obtained in step "2" to obtain strips,
[0042] 5. Round the strips to get pills.
[0043] 6. Dry the balls,
[0044] 7. Sieve the dried balls to obtain balls within the required particle size range.
[0045] 8. The balls prepared in step "6" can be optionally coated with Opadry85G68918 stomach-soluble coating powder. Coating was performed using fluidized bed bottom spray coating.
[0046] 9. Pour the balls into the bag.
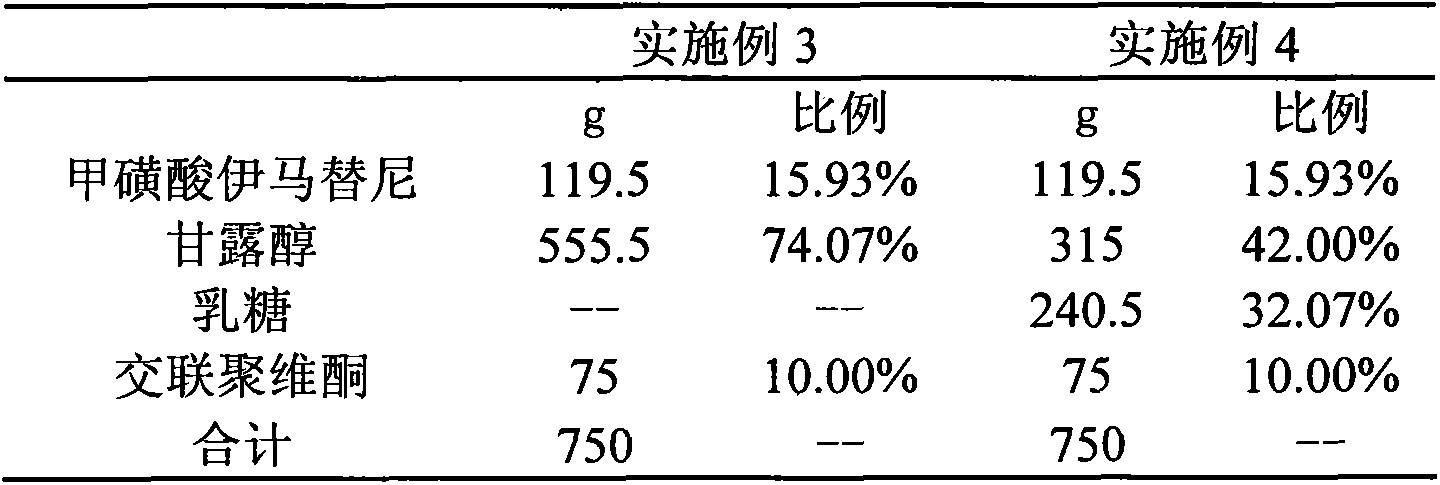
Embodiment 3~4
[0048]
[0049] Preparation Process:
[0050] 1. Mix imatinib mesylate, mannitol, lactose, and crospovidone,
[0051] 2. Pour the mixed material into a centrifugal spray granulator, spray 95% ethanol solution, and prepare pellets.
[0052] 3. Dry the balls obtained in step "2",
[0053] 4. Sieve the balls obtained in step "3" to obtain balls within the required particle size range.
[0054] 5. Fill the prepared balls into the bag.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com