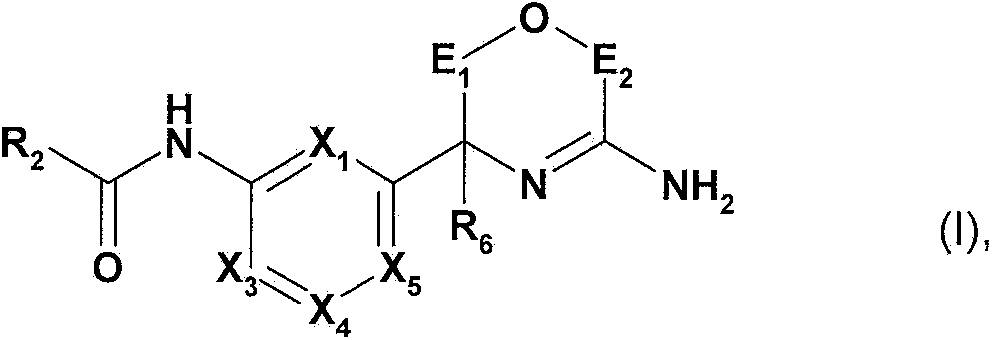
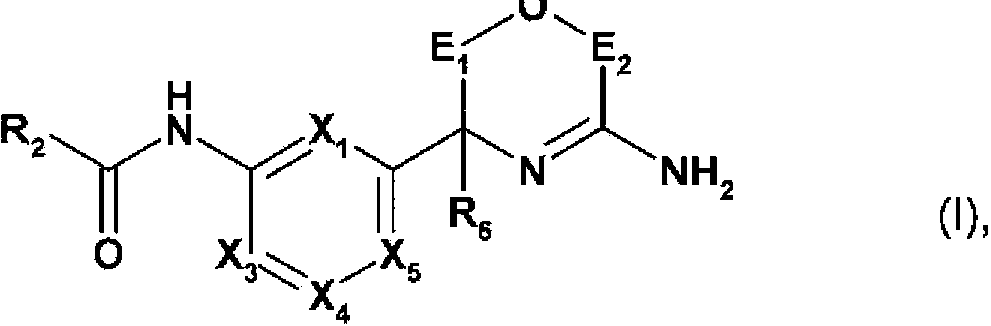
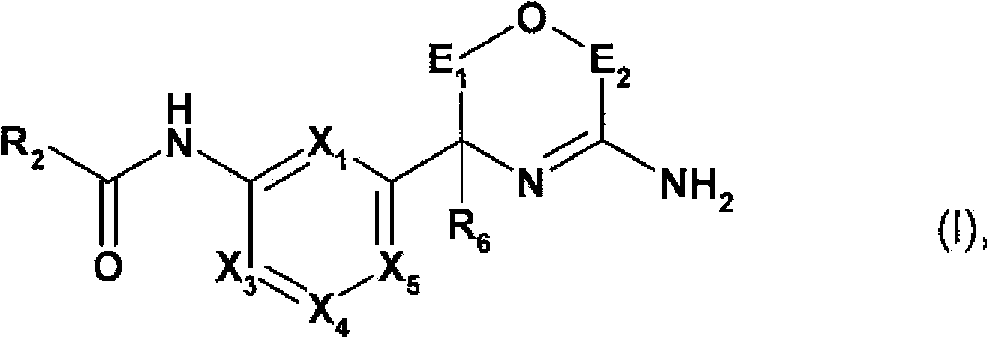
Novel heterocyclic derivatives and their use in the treatment of neurological disorders
A compound, heteroaryl technology, applied in the field of new heterocyclic derivatives and its application in the treatment of neurological diseases, can solve the problem of different substrate specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0371] In another aspect, the present invention relates to a method for the preparation of a compound of formula I in free form or in salt form, the method comprising:
[0372] a) a compound of the following formula in free form or in salt form:
[0373]
[0374] where X 1 、X 3 、X 4 、X 5 , R 6 ,E 1 and E 2 As defined in formula I, PG is a protecting group, reacting with the following formula compound in free form or salt form:
[0375]
[0376] where R 2 As defined in formula I, L is a leaving group, such as a hydroxyl group,
[0377] b) making the following formula compound in free form or salt form:
[0378]
[0379] where X 1 、X 3 、X 4 、X 5 , R 6 ,E 1 and E 2 As defined in formula I, Hal is a halogen, such as bromine, and PG is a protecting group,
[0380] Reacts with a compound of the following formula in free or salt form:
[0381]
[0382] where R 2 As defined in Formula I,
[0383] c) make the following formula compound of free form or sa...
Embodiment 1
[0601] Example 1: 5-Bromo-pyridine-2-carboxylic acid [6-((R)-5-amino-3-methyl-3,6-dihydro-2H-[1,4]-oxazine-3- base)-pyridin-2-yl]-amide
[0602]
[0603] a) 5-(6-bromo-pyridin-2-yl)-5-methyl-imidazolidine-2,4-dione
[0604] To 1-(6-bromo-pyridin-2-yl)-ethanone (CAS 49669-13-8, 8.75g, 43.7mmol) and potassium cyanide (4.27g, 65.6mmol) in ethanol / water (40.0 / 26.7 ml) solution was added ammonium carbonate (21.02 g, 219.0 mmol). The reaction mixture was stirred in an autoclave at 100°C for 17h, then water, 1Maq.NaHCO 3 soln. and EtOAc dilution. The phases were separated, and the aqueous phase was washed with EtOAc, Et 2 O and DCM re-extracted. The combined organic phases were dried over sodium sulfate, filtered and concentrated to afford the title compound as an off-white solid which was used directly in the next step without further purification.
[0605] HPLC Rt H4 = 0.62min; ESIMS: 270, 272 [(M+H) + ]; 1 H NMR (400MHz, DMSO-d 6 ): δ10.86 (br s, 1H), 8.48 (s, 1H), 7...
Embodiment 2
[0632] Example 2: 5-Bromo-pyridine-2-carboxylic acid [6-(5-amino-3-methyl-3,6-dihydro-2H-[1,4]-oxazin-3-yl)-pyridine -2-yl]-amide
[0633]
[0634] The racemate of Example 1 can be prepared according to the method used in Example 1, the synthesis being accomplished using the racemic mixture obtained from Example 1, step j), which has the same analytical data.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com