Reagent, and its application in detection of divalent zinc ions
A reagent, pyridoxal technology, applied in the field of optical probes, can solve problems such as zinc ions are not applicable, achieve high sensitivity and selectivity, simple detection process, and simple detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of pyridoxal fluorescein hydrazone: Dissolve 1mmoL of fluorescein hydrazide and 2mmoL of pyridoxal in 20mL of ethanol, add dropwise 1mmoL of anhydrous acetic acid, reflux for 2 hours, and reconstitute with 1:1 methanol and petroleum ether. The yellow pyridoxal fluorescein acylhydrazone was obtained by crystallization, and the yield was 65%.
[0024] Characterization of pyridoxal fluorescein acylhydrazone:
[0025] 1 H NMR (300MHz, 25°C, DMSO-d 6 ):δ11.75(s,1H),10.11(s,2H),9.09(s,N=C-H,1H),8.05(s,1H),7.99(t,1H),7.67(m,2H), 7.17(t,1H),6.71(s,2H),6.54(m,2H),6.48(m,2H),4.41(s,2H),2.61(s,3H); 13 C NMR (75MHz, DMSO-d 6 ): δ163.6, 158.4, 151.3, 150.4, 143.0, 142.0, 135.9, 137.00, 134.7, 129.7, 129.0, 127.80, 127.6, 126.5, 123.4, 112.3, 107.4, 102.0, 64.5, 57.7, 14 .25[PFH+H] + ;Elemental analysis (calcd.%) for C 28 h 21 N 3 o 6 :C,67.87;H,4.27;N,8.48;Found:C,67.85;N,8.52;H,4.21.
Embodiment 2
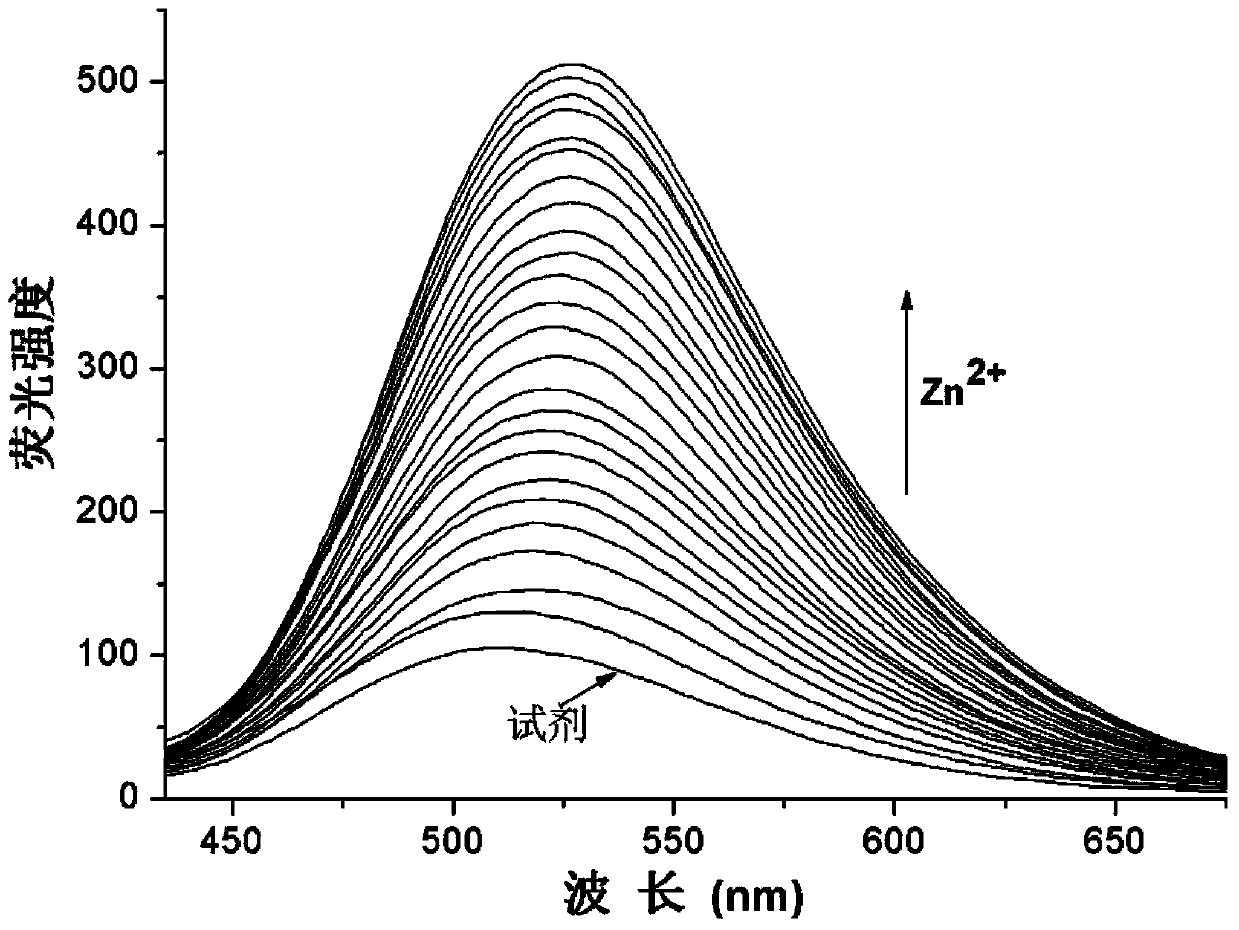
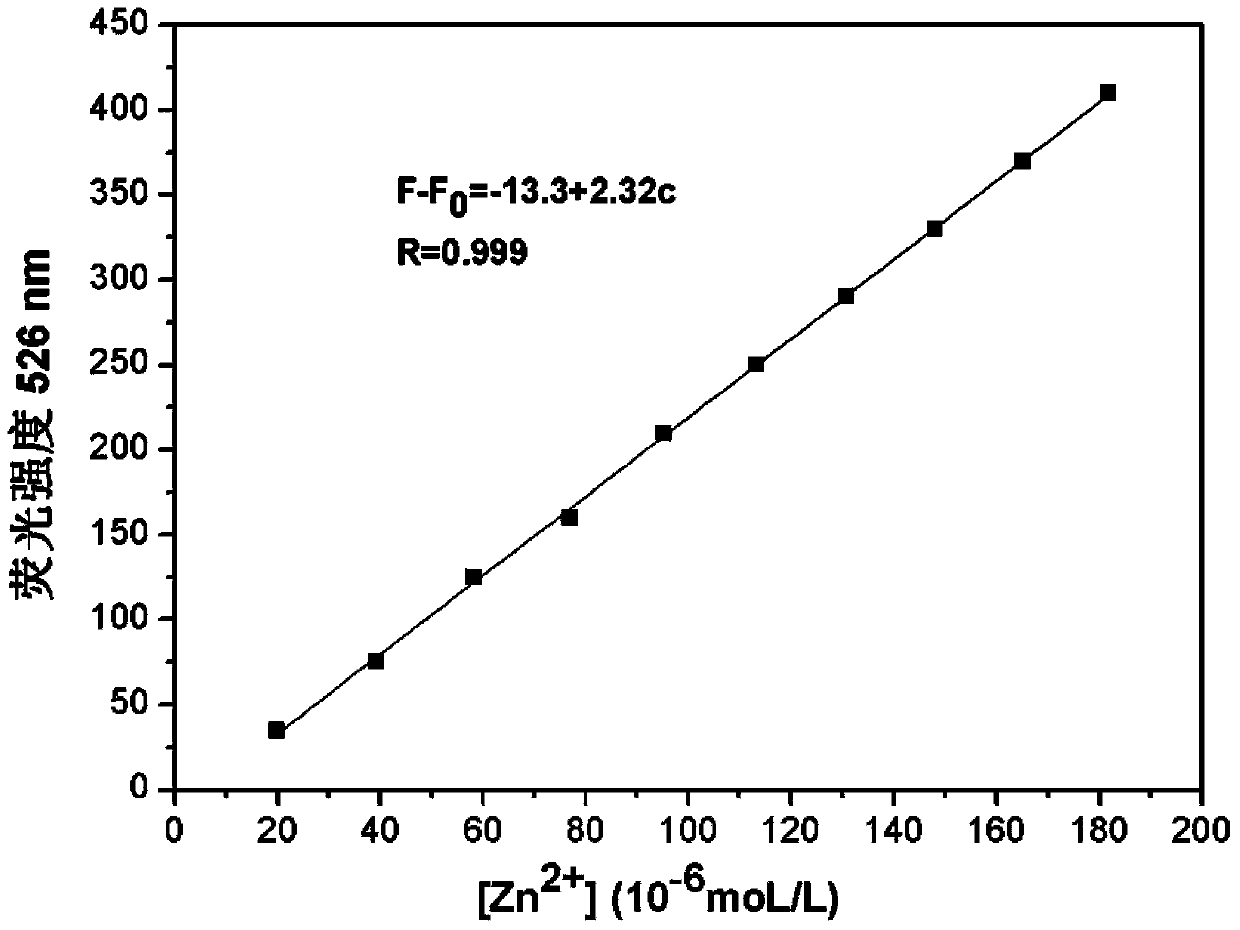
[0027] Prepare HEPES (10mM) buffer solution with pH=7.0, prepare 2mM divalent zinc ion aqueous solution, and prepare 2mM pyridoxal fluorescein acylhydrazone solution with ethanol; mix 2mL of HEPES buffer solution and 8μL pyridoxal fluorescein acylhydrazone Hydrazone ethanol solution was added to a clean fluorescence cuvette, detected on a fluorescence spectrophotometer, along with Zn 2+ The addition of , the fluorescence intensity at 526nm gradually increased. Fluorescence emission map see figure 1 .
Embodiment 3
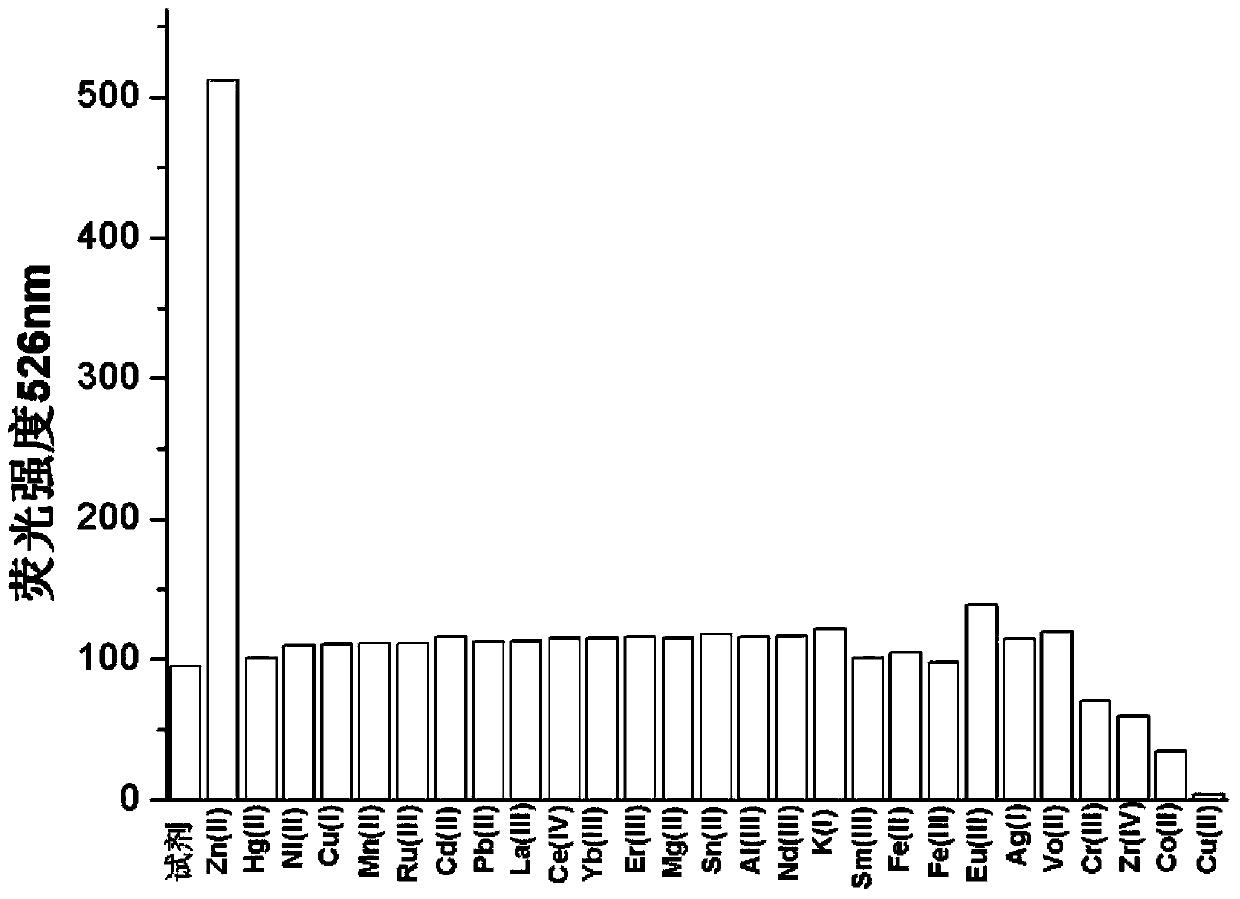
[0029] Prepare a HEPES buffer solution with a pH=7.0 and a concentration of 10mM, and prepare a 2mM pyridoxal fluorescein acylhydrazone solution with ethanol; add 2mL of HEPES buffer solution and 8μL of pyridoxal fluorescein to each of the 27 fluorescent cuvettes. Su-acylhydrazone ethanol solution, then add 25 molar equivalents of Zn 2+ , and 100 molar equivalents of various other analytes (Hg 2+ , Ni 2+ ,Cu + , Mn 2+ , Ru 3+ ,Cd 2+ ,Pb 2+ ,La 3+ , Ce 4+ ,Yb 3+ ,Er 3+ ,Mg 2+ ,Sn 2+ ,Al 3+ ,Nd 3+ , K + ,Sm 3+ , Fe 2+ , Fe 3+ , Eu 3+ , Ag + , Vo 2+ ,Cr 3+ ,Zr 4+ ,Co 2+ ,Cu 2+ , detected on a fluorescence spectrophotometer, draw the histogram of the 526nm fluorescence intensity corresponding to different analytes, and obtain the fluorescence emission diagram (see figure 2 ), Zn 2+ The fluorescence intensity of pyridoxal fluorescein acylhydrazone changed from 99 to about 510, and other analytes basically did not cause changes in the fluorescence intensit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com