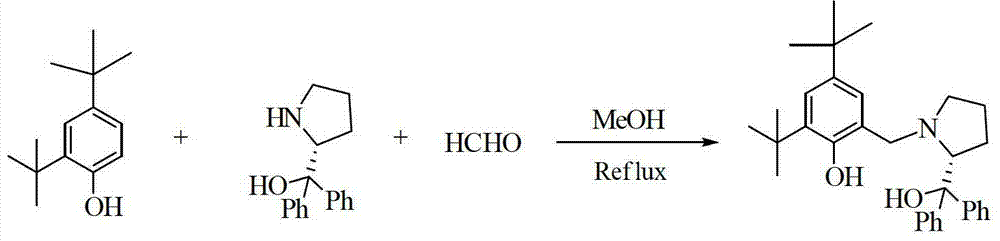Preparation method of chiral epoxy compound
A technology of epoxy compounds and rare earth compounds, applied in chemical instruments and methods, chemical/physical processes, organic compounds/hydrides/coordination complex catalysts, etc., can solve problems that have not been seen yet, and reach the scope of substrate adaptation Wide range, simple synthesis, high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
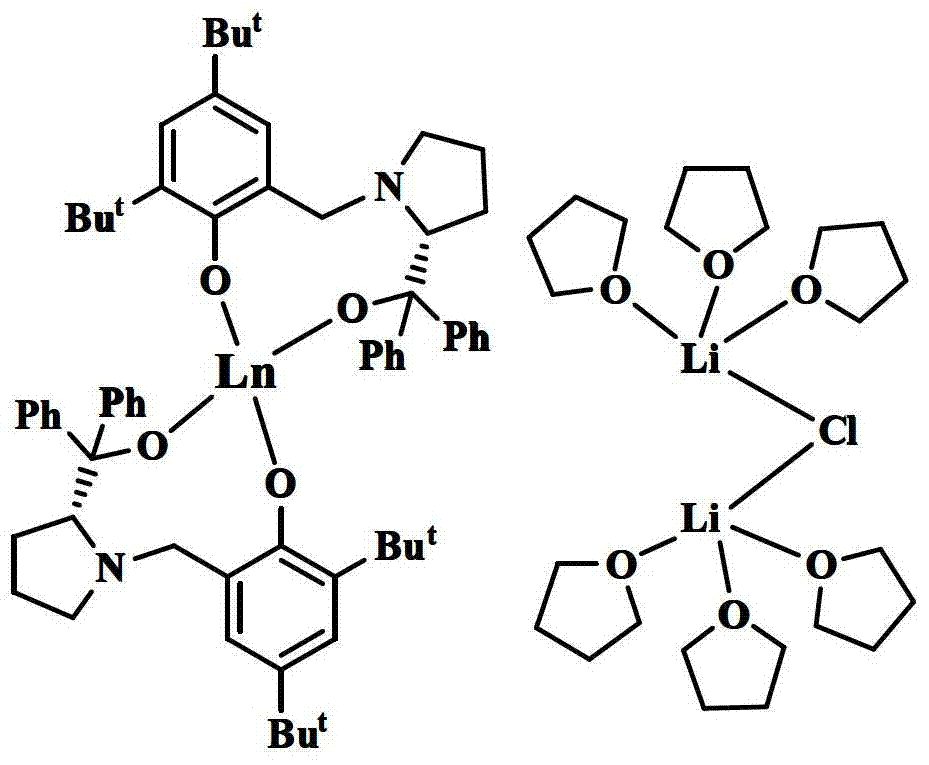
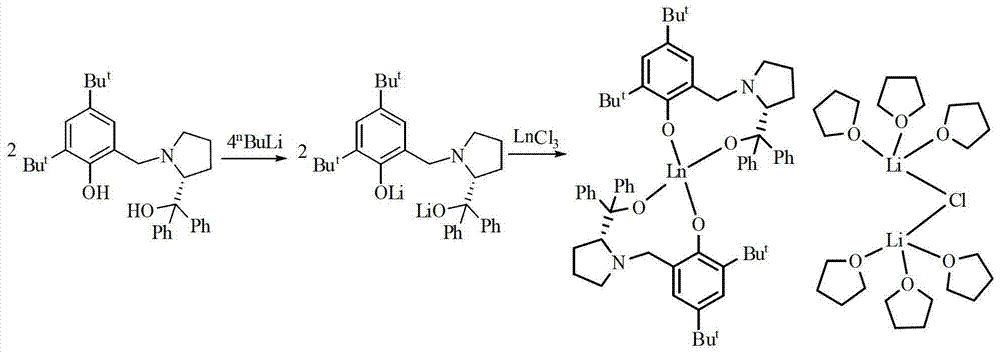
[0056] Example 1: Using the above prepared [YL 2 ][{(THF) 3 Li} 2 (μ-Cl)] Catalyzes the asymmetric epoxidation reaction of α,β-unsaturated ketones:
[0057] In the reaction flask that has been dehydrated and deoxygenated, add chalcone (ie chalcone without substituents, the same as in Examples 2 to 5) 0.0625 g (0.3 mmol) and 0.048 g (0.03 mmol) of the catalyst under the protection of argon. Millimoles), add 2.0 ml of tetrahydrofuran, stir in a constant temperature bath set at 25°C for 5 minutes, add 0.065 ml of tert-butyl hydroperoxide (5.5 mmol / ml of n-decane solution), continue at 25°C After 4 hours of reaction, the reaction was terminated with saturated aqueous sodium sulfite solution.
[0058] The product was separated by silica gel column, and eluted with the eluent of ethyl acetate: petroleum ether=1:30 to obtain 57.80 mg of epoxidized chalcone with a yield of 95%. Enantioselectivity was determined by chiral HPLC, Daicel Chiralpak OJ column, eluent i-PrOH / hexane (10 / 90), flow...
Embodiment 2
[0059] Example 2: Using the above prepared [YbL 2 ][{(THF) 3 Li} 2 (μ-Cl)] Catalyzes the asymmetric epoxidation reaction of α,β-unsaturated ketones:
[0060] In a reaction flask that has undergone dehydration and deoxygenation, 0.104 g (0.5 mmol) of chalcone and 0.076 g (0.05 mmol) of catalyst are added under the protection of argon, and 3.2 ml of tetrahydrofuran is added. The temperature is set at 0°C. After stirring in a constant temperature bath for 20 minutes, add 0.11 ml of tert-butyl hydroperoxide (5.5 mmol / ml n-decane solution), continue the reaction at 0°C for 12 hours, and terminate with saturated sodium sulfite aqueous solution.
[0061] The product was separated by a silica gel column, and eluted with an eluent of ethyl acetate:petroleum ether=1:30 to obtain 78.4 mg of epoxidized chalcone with a yield of 70%. Enantioselectivity was determined by chiral HPLC, Daicel Chiralpak OJ column, eluent i-PrOH / hexane(10 / 90), flow rate 1.0mL / min, ee value 89%.
Embodiment 3
[0062] Example 3: Using the above-prepared [SmL 2 ][{(THF) 3 Li} 2 (μ-Cl)] Catalyzes the asymmetric epoxidation reaction of α,β-unsaturated ketones:
[0063] In the reaction flask after dehydration and deoxygenation, add chalcone 0.0625 g (0.3 mmol), catalyst 0.048 g (0.03 mmol), add 1.9 ml of tetrahydrofuran, set the temperature at 25 ℃ under the protection of argon After stirring in a constant temperature bath for 5 minutes, add 0.065 ml of tert-butyl hydroperoxide (5.5 mmol / ml of n-decane solution), continue the reaction at 25°C for 4 hours, and terminate with saturated sodium sulfite aqueous solution.
[0064] The product was separated by silica gel column, and eluted with the eluent of ethyl acetate: petroleum ether=1:30 to obtain 63.2 mg of epoxidized chalcone, with a yield of 94%. Enantioselectivity was determined by chiral HPLC, Daicel Chiralpak OJ column, eluent i-PrOH / hexane (10 / 90), flow rate 1.0 mL / min, ee value 15%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com