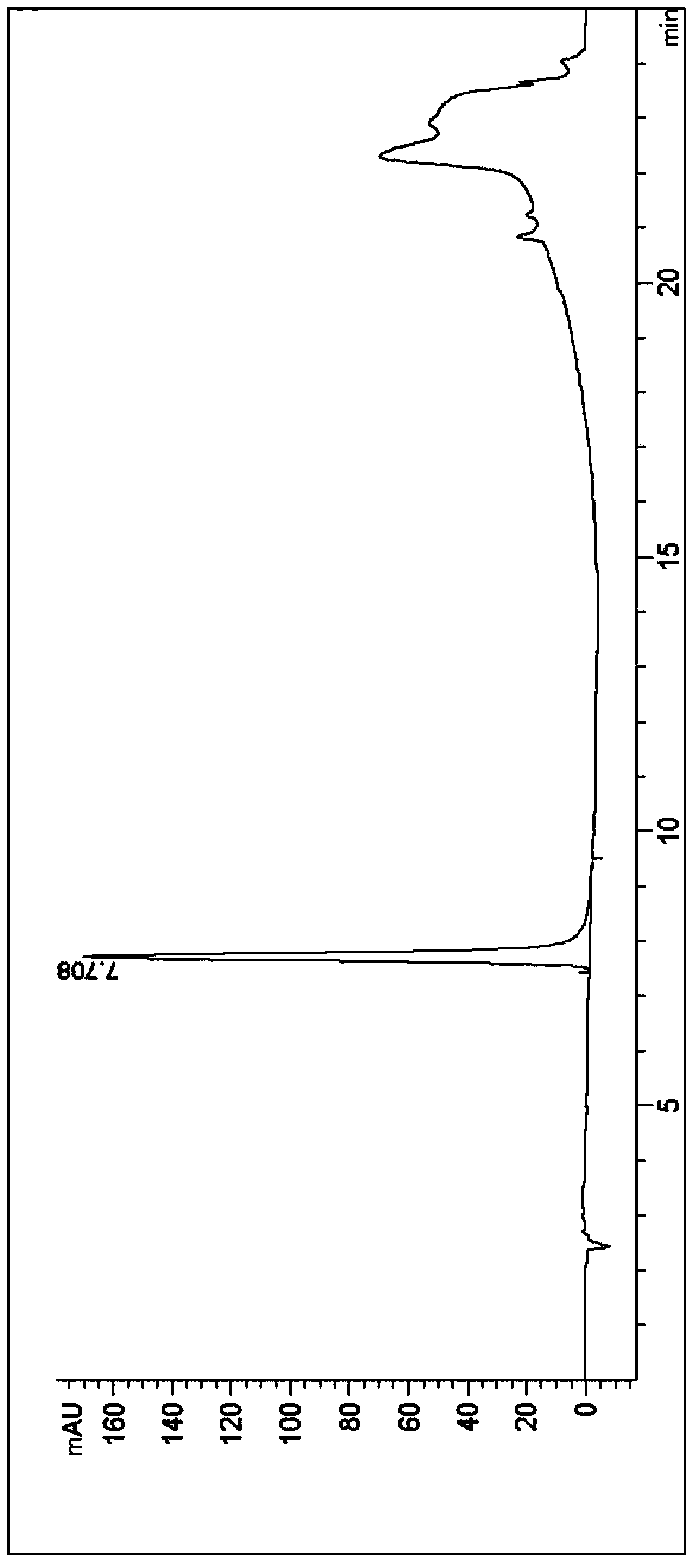
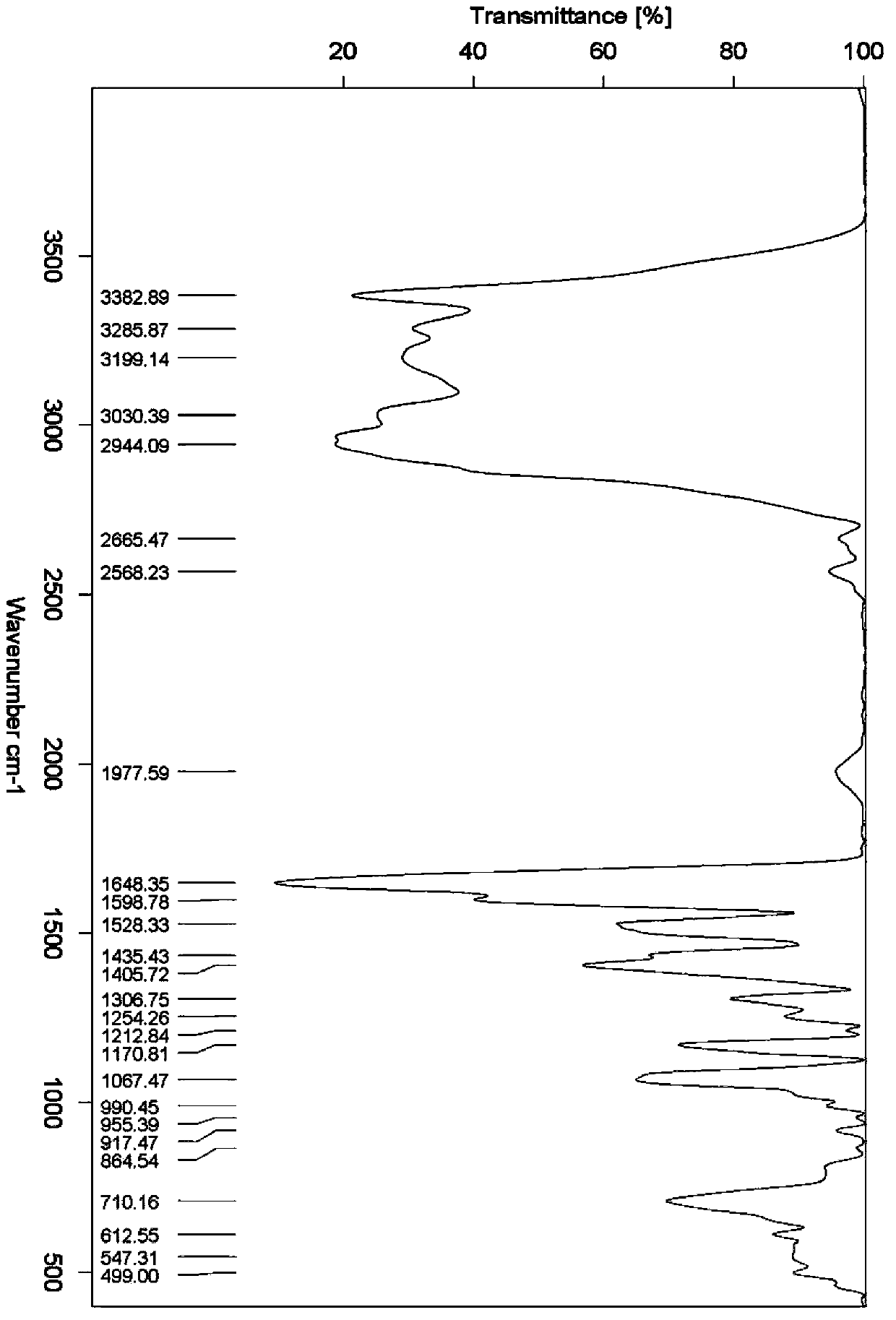
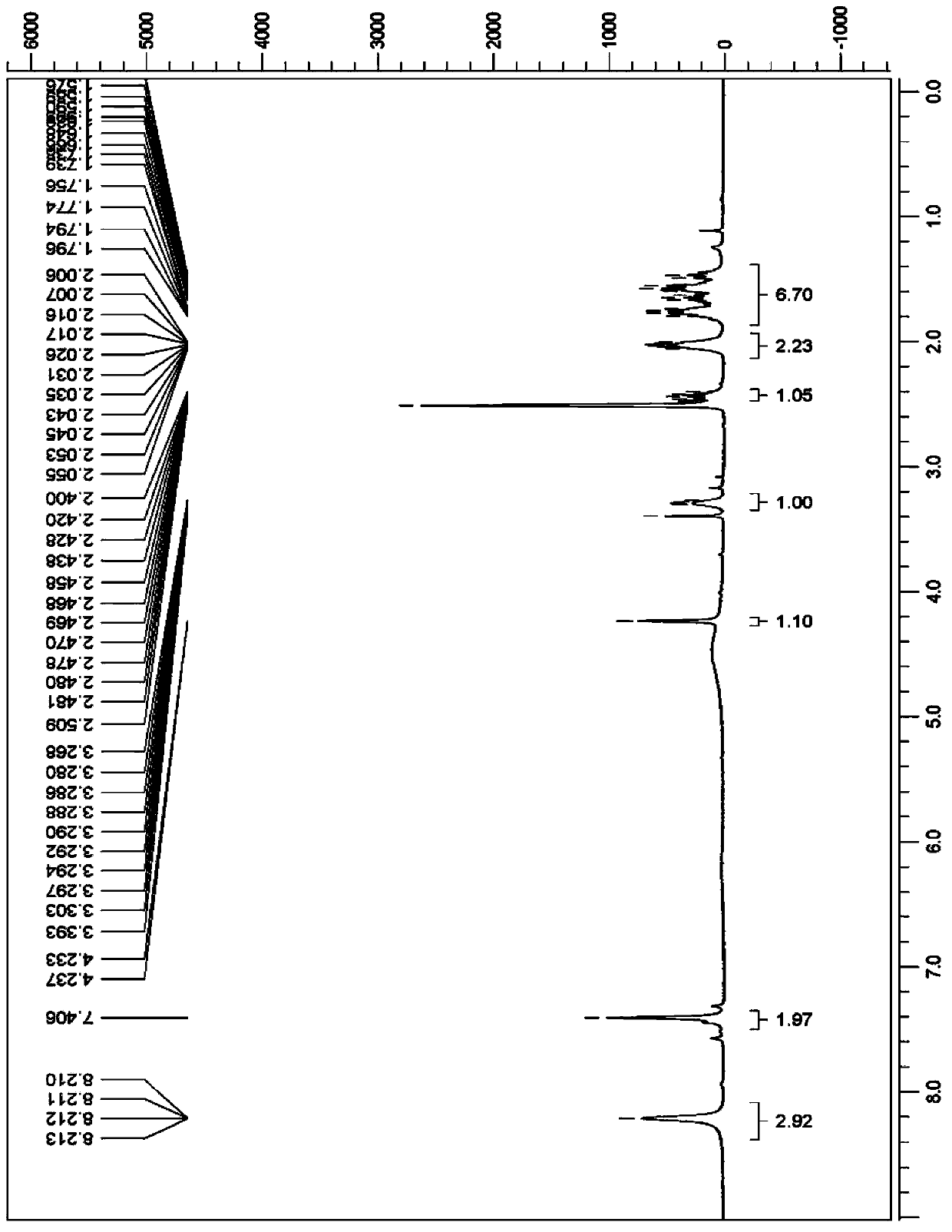
Synthesis method of beta-amino-alpha-hydroxycyclohexyl butyl aluminum hydrochloride
A technology of hydroxycyclobutylbutanamide and a synthetic method, which is applied in the field of synthesis of boceprevir intermediates, can solve problems such as low product purity and yield, unfavorable industrial production, and harsh conditions, so as to improve yield and The effects of purity, reduced need for auxiliary raw materials, and fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Dissolve 150g of Boc-glycine tert-butyl ester in 1.5L of THF, stir for 0.5h, and cool down to -30°C; slowly add 648ml of LDA dropwise to the system, keep stirring for 0.5h; add 96.7g of cyclobutane to the system dropwise After dropping the system, the system was kept at -30°C for 2 hours; 1.5L of 10% ammonium chloride aqueous solution and 1.5L of MBTE were added to the system, extracted and separated, the aqueous phase was extracted with 1.5L of MBTE, the organic phases were combined, and Wash with 1.5L saturated brine, then wash with 300g anhydrous NaSO 4 Dry, filter with suction, and concentrate the filtrate under reduced pressure until solvent-free to obtain tert-butyl 2-tert-butoxycarbonyl amino acid-3-cyclobutylpropanoate with a yield of 90.0%.
[0040] Dissolve 150g of 2-tert-butoxycarbonyl amino acid-tert-butyl 3-cyclobutylpropanoate in 1.5LTHF, under nitrogen protection, warm to -20°C, slowly add 551mL of 1.1eq of diisobutylaluminum hydride dropwise, After the ...
Embodiment 2
[0047] Dissolve 150g Boc-glycine tert-butyl ester in 2.0L methyl tert-butyl ether, stir for 20 minutes, and cool down to -40°C; slowly add 1000ml HMDSLi dropwise to the system, keep stirring for 20 minutes; add dropwise to the system Add 136 g of cyclobutylchloromethane, and after dropping the system, continue to keep the temperature at -40°C for 2 hours; add 2.0L of 10% ammonium chloride aqueous solution and 2.0L of MBTE to the system, extract and separate liquids, and then extract the aqueous phase with 2.0L of MBTE, The organic phases were combined, washed with 2.0L saturated brine, and then washed with 450g anhydrous NaSO 4 Dry, filter with suction, and concentrate the filtrate under reduced pressure until solvent-free to obtain tert-butyl 2-tert-butoxycarbonyl amino acid-3-cyclobutylpropanoate with a yield of 91.6%.
[0048] Dissolve 150g of 2-tert-butoxycarbonyl amino acid-tert-butyl 3-cyclobutylpropanoate in 2.0L of methyl tert-butyl ether, under nitrogen protection, wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com