Fingerprint model for liver cancer serum characteristic protein detection and preparation method thereof
A fingerprint and characteristic protein technology, which is applied in the field of protein fingerprint detection, can solve the problems that have not been reported, achieve the effect of comprehensive population range, overcome poor repeatability and low stability, and reduce the fatality rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
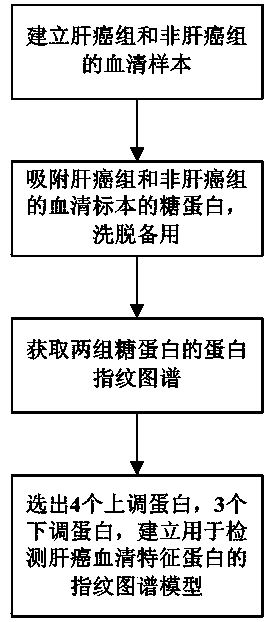
[0023] The present invention is used for the preparation method of the fingerprint atlas model that detects liver cancer serum characteristic protein, comprises the following steps:
[0024] 1) Serum samples from patients with primary liver cancer related to hepatitis B virus that were clearly diagnosed by pathological and DNA examinations were collected as serum samples of the liver cancer group; serum samples from patients with liver cirrhosis caused by confirmed hepatitis B virus were confirmed Serum from patients with chronic hepatitis B without liver cirrhosis and those from healthy subjects determined by physical examination were used as serum specimens of the non-liver cancer group, and were cryogenically frozen for future use.
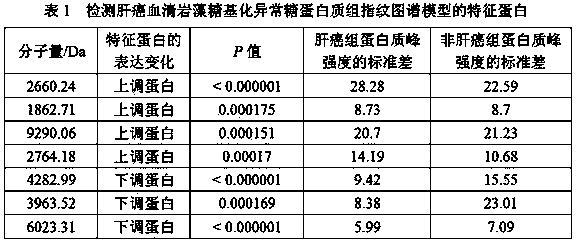
[0025] 2) MB-LAC LCA magnetic beads, which can specifically adsorb fucosylated abnormal glycoproteins, were used to adsorb the glycoproteins of the serum samples of the above-mentioned liver cancer group and non-liver cancer group, and then the ...
Embodiment 1
[0035] Take 2ml of venous blood from the patient to be tested without anticoagulation. Place at room temperature for 30 min to 1 h. Centrifuge at room temperature at 2500 rpm for 5 min, take equal aliquots of serum and store in a -80°C refrigerator. During the experiment, the specimens were taken out from the -80°C refrigerator and thawed in ice for later use. Take out one tube of MB-LAC LCA magnetic bead suspension from the 4°C refrigerator and pipette carefully to suspend the magnetic beads completely and evenly in the liquid phase; place the 200 μl sample tube on the well plate, add 2 μl magnetic beads ( MB) and 100 μl magnetic bead washing buffer (WB1), transfer the sample tube to the magnetic rack, let the magnetic beads adhere to the wall for 20 s, separate the magnetic beads from the liquid, and suck up the liquid after the liquid is clarified; Aspirate the supernatant; repeat once to ensure that the suspension is completely absorbed; put the sample tube on the orifice...
Embodiment 2
[0037] Take 2ml of venous blood from the patient to be tested without anticoagulation. Place at room temperature for 30 min to 1 h. Centrifuge at room temperature at 2500 rpm for 5 min, take equal aliquots of serum and store in a -80°C refrigerator. During the experiment, the specimens were taken out from the -80°C refrigerator and thawed in ice for later use. Take out one tube of MB-LAC LCA magnetic bead suspension from the 4°C refrigerator and pipette carefully to suspend the magnetic beads completely and evenly in the liquid phase; place the 200 μl sample tube on the well plate, add 2 μl magnetic beads ( MB) and 100 μl magnetic bead washing buffer (WB1), transfer the sample tube to the magnetic rack, let the magnetic beads adhere to the wall for 20 s, separate the magnetic beads from the liquid, and suck up the liquid after the liquid is clarified; Aspirate the supernatant; repeat once to ensure that the suspension is completely absorbed; put the sample tube on the orifice...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com