Kit for detecting furazolidone metabolin and method thereof
A technology for furazolidone and metabolites, which is applied in the field of kits for detecting furazolidone metabolites, can solve the problems that the detection limit of the detection method cannot be met, the processing process is complicated, the degree of instrumentation is high, and the detection time is short, the operation is simple, and the sensitivity is achieved. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 detects the composition of the kit of furazolidone metabolite
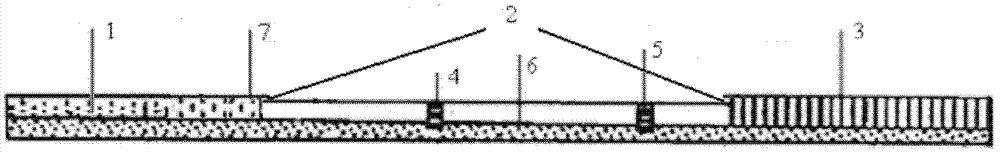
[0042] 1. Test paper (called test strip) ( figure 1 )
[0043] The test paper is composed of a bottom plate, a sample absorption pad, a reaction film, a water absorption pad, and a protective film;
[0044] The sample absorbent pad 1, the reaction film 2, the water absorbent pad 3 and the protective film 7 are pasted on the bottom plate 6 in sequence, the end of the sample absorbent pad is connected with the reaction film, the end of the reaction film is connected with the water absorbent pad, and the sample absorbent pad The beginning is aligned with the beginning of the bottom plate, and the end of the absorbent pad is aligned with the end of the bottom plate;
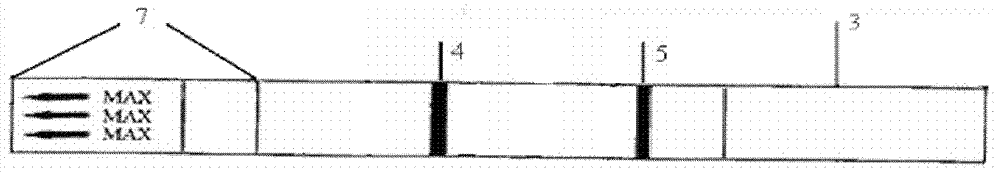
[0045] The test paper is pasted with a protective film, and the protective film 7 is covered on the sample absorption pad, which is the detection end, and the word MAX is printed on it ( figure 2 );
[0046] There is a detection...
Embodiment 3
[0099] The detection of furazolidone metabolite in the animal tissue of embodiment 3
[0100] 1. Sample pretreatment
[0101]Take (5.0±0.05)g homogeneous sample to a 50ml polystyrene centrifuge tube, add 5ml 10% trichloroacetic acid, then add 100μl derivatization reagent (add methanol to the reagent bottle containing 151mg 2-nitrobenzaldehyde Dissolve to 10ml), shake fully for 3min, and place at 60°C for 1.5h; take out and add 1ml 0.5mol / L dipotassium hydrogen phosphate solution, 1.5ml 2mol / L sodium hydroxide solution and 10ml ethyl acetate, shake for 10s, Slightly shake the sample with high fat content for 8-10 times; centrifuge at room temperature (20-25°C) for 3000g or more; take 8ml of ethyl acetate phase into a 10ml dry glass test tube, and dry it in a water bath at 50-60°C under nitrogen flow. Add 1ml of n-hexane, vortex for 30s with a vortex, then add 0.6ml of 0.2mol / L phosphate buffer, vortex for 10s to mix thoroughly; above 3000g, centrifuge at room temperature (20-2...
Embodiment 4
[0108] The determination of embodiment 4 kit technical parameter
[0109] 1. Sensitivity test
[0110] The furazolidone metabolite standard (purchased from Sigma) was diluted to 0.5, 1, 2 μg / L; the diluent used was pH7.2, 0.2mol / L phosphate buffer.
[0111] Tested with the kit, the results are: when the concentration of the standard furazolidone metabolite is 0.5 μg / L, two red bands visible to the naked eye appear on the test strip, which is negative; the concentration of the standard furazolidone metabolite is 1 and 2 μg / L, the quality control area of the test strip develops color, but the detection area does not develop color, which is positive, indicating that the sensitivity of this kit to detect furazolidone metabolites is 1 μg / L.
[0112] 2. False positive rate, false negative rate test
[0113] Take 20 positive samples of pork, chicken, fish, and shrimp with known furazolidone metabolite content greater than 1 μg / L and 20 negative samples of pork, chicken, fish, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com