8-chlorotheophylline preparation method
A technology of chlorotheophylline and theophylline, which is applied in the field of preparation of 8-chlorotheophylline, can solve the problems of chlorine gas influx, temperature, and pressure, which are difficult to control, and achieve the advantages of environmental protection, improved utilization rate, and easy industrialization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
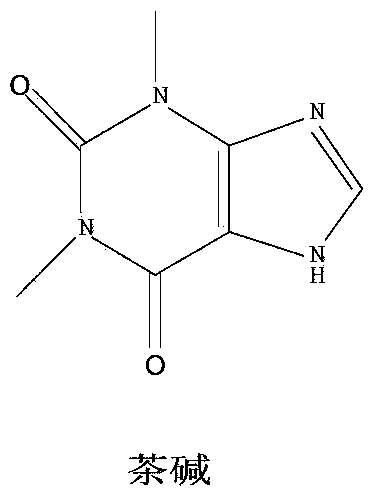
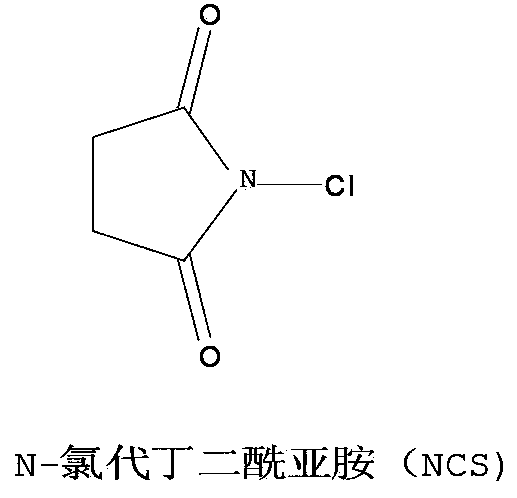
[0036] Add 18g of theophylline (provided by Shanghai Bandai Pharmaceutical Co., Ltd., 0.1mol) into a 500ml three-necked flask, add 150ml of water, stir and heat up to 70°C to completely dissolve, then add dropwise N-chlorosuccinimide (NCS, purchased from Nanjing Suru Chemical Co., Ltd., 0.11mol) 14.63g aqueous solution 100ml, 30 minutes after the start of the reaction, directly sample the reaction solution with a sampling capillary to monitor the reaction process.
[0037] TLC 254 Thin-layer chromatography detection, the specific method is as follows:
[0038] Two TLC thin-layer chromatography plates (Taizhou Luqiao Sijia Biochemical Plastic Factory, silica gel chromatography plate (3×10cm), GF254), developer: dichloromethane: methanol = 10:1, capillary spotting tubes.
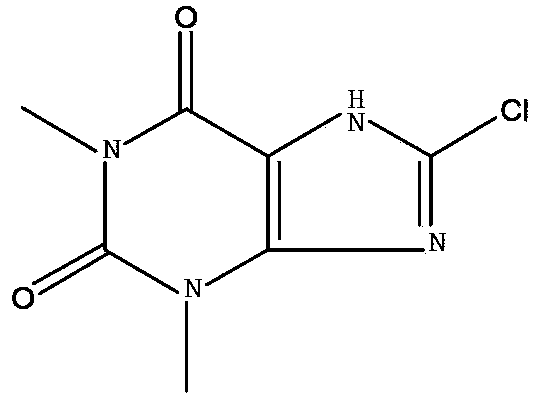
[0039] Compare raw material point Rf 茶碱 =0.65, Rf 8-氯茶碱 =0.40 If TLC shows the presence of theophylline, continue to add NCS dropwise, and react for 2 hours until TLC shows that theophylline raw material ...
Embodiment 2
[0045] Add 18g of theophylline (provided by Shanghai Bandai Pharmaceutical Co., Ltd., 0.1mol) into a 500ml three-necked flask, add 150ml of water, stir and heat up to 80°C to dissolve, and add N-chlorosuccinimide (NCS, purchased from Nanjing) dropwise at this temperature. Suru Chemical Co., Ltd., 0.13mol) 17.3g aqueous solution 100ml, dropwise added in 30 minutes, reacted at 80°C for 2 hours, controlled pH between 6 and 7, lowered to room temperature 20°C, a large amount of off-white solid precipitated.
[0046] Filtration, the filter cake was dissolved in 100ml of 5% NaoOH aqueous solution and heated to 80°C, cooled to room temperature, adjusted to pH 3-3.5 with 10% dilute hydrochloric acid and stirred for 0.5 hours, the solid precipitated, filtered, washed with water, dried, and dried to obtain a white solid 17.2 g. Yield 82%, HPLC 99.65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com