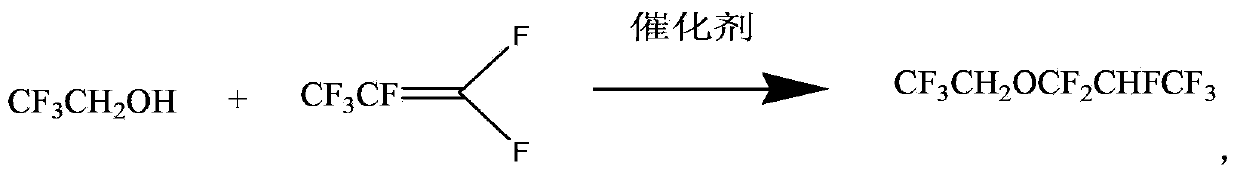
Preparation of 2,2,2- trifluoroethyl-1,1,2,3,3,3-hexafluoroisopropyl ether
A technology of hexafluoropropyl ether and trifluoroethyl, applied in the field of preparation of 2,2,2-trifluoroethyl 1,1,2,3,3,3-hexafluoropropyl ether, can solve Palladium is expensive, not suitable for large-scale industrial production, and has toxicity, etc., to achieve the effect of convenient post-processing, mild conditions, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of CF3CH2OCF2CHFCF3 under the optimal conditions of embodiment 1
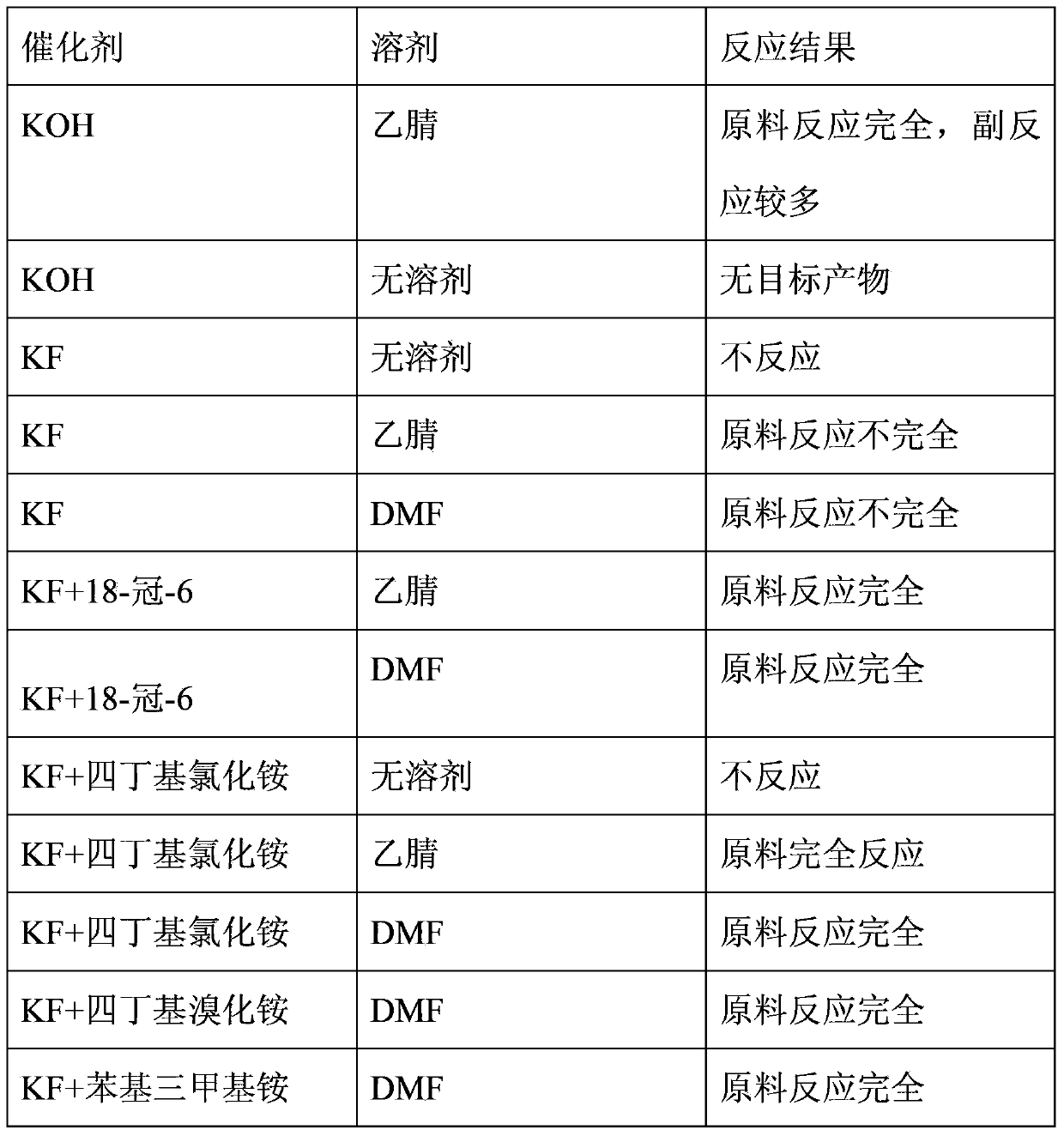
[0023] Take 330ml of DMF and add it into a three-necked flask, add 5.3g of KF and 25g of tetrabutylammonium chloride into it and stir to dissolve. Add 330ml CF3CH2OH, then pass through perfluoropropene gas, and control the reaction temperature to 40°C with an oil bath until the reaction is complete. After post-treatment, rectification under normal pressure to obtain the final product 2,2,2-trifluoroethyl 1,1,2,3,3,3-hexafluoropropyl ether with a boiling point of 71.5-73°C and a purity of 99.7%. Yield 70-80%.
Embodiment 2
[0024] The preparation of CF3CH2OCF2CHFCF3 under embodiment 2KF catalyzed acetonitrile as solvent condition
[0025] Weigh 0.04g KF into a three-necked flask, add 60ml of acetonitrile and stir to dissolve, then add 7.2ml of CF3CH2OH, pass perfluoropropylene gas to evacuate the air, and then pass perfluoropropylene gas to react at 40°C under airtight conditions. There are surplus raw materials, and the yield is 40-50%.
Embodiment 3
[0026] The preparation of CF3CH2OCF2CHFCF3 under embodiment 3KF catalyzed DMF as solvent condition
[0027] As in the method of Example 2, 0.04g KF, 60ml acetonitrile and 7.2ml CF3CH2OH were weighed for reaction. There are surplus raw materials, and the yield is 40-50%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com