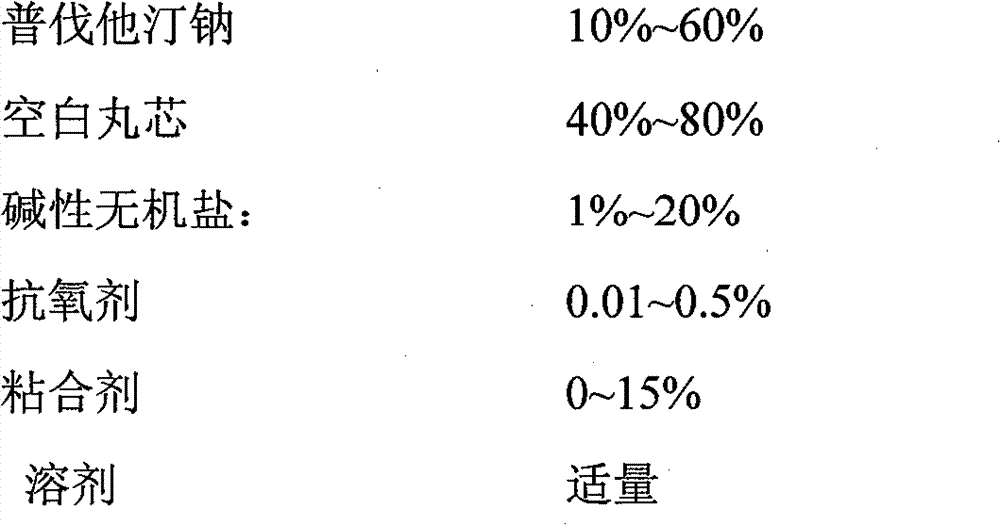
Preparation method of fenofibrate and pravastatin sodium compound preparation
A technology of pravastatin sodium and fenofibrate, which is applied in the field of pharmaceutical preparations and can solve problems such as difficult industrial production, cumbersome design and preparation methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
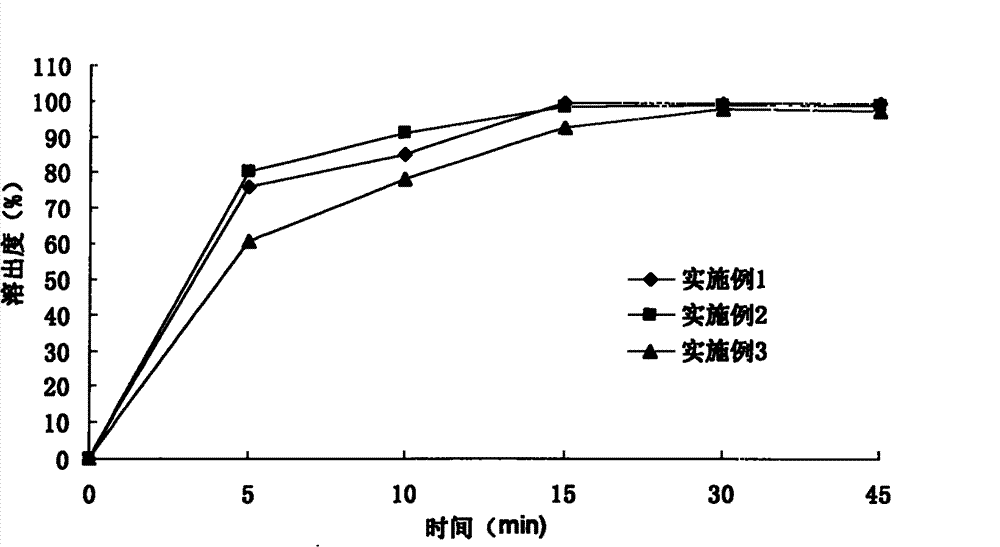
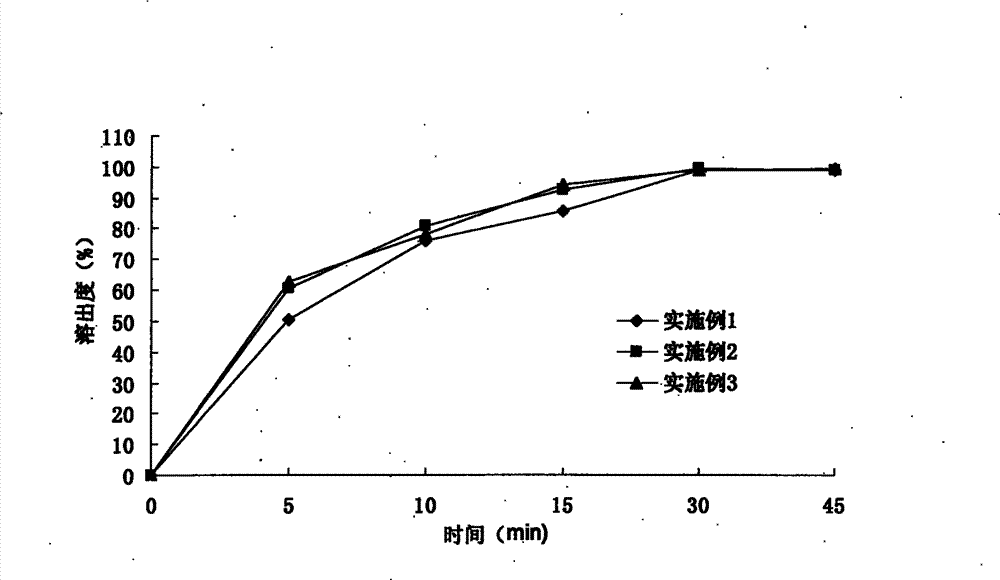
Examples
Embodiment 1
[0065] Prescription of pravastatin sodium pellets:
[0066]
[0067] Prescription of fenofibrate semi-solid granules:
[0068]
[0069]
[0070] Preparation:
[0071] Preparation of pravastatin sodium pellets: Dissolve pravastatin sodium, sodium bicarbonate, vitamin C, and povidone K30 in 50% ethanol solvent, add blank pellet cores to the fluidization chamber, adjust the air volume, and make most of the micropellets The pills are blown up in the fluidization chamber, and the physicochemical pressure and the flow rate of the constant-flow pump are adjusted to make the drug solution atomized well, and then the drug is sprayed and the drug is applied. After the drug is applied, the hot air is used for 5 minutes to dry. After drying, continue to coat in the same way as applying the medicine, and you get it.
[0072] (2) Preparation of fenofibrate semi-solid granules: dissolving fenofibrate in glycerol acetate; heating and melting macrogol glyceryl laurate and macrogol ...
Embodiment 2
[0075] Prescription of pravastatin sodium pellets:
[0076]
[0077] Prescription of fenofibrate solid particles:
[0078]
[0079] Preparation:
[0080] Preparation of pravastatin sodium pellets: Dissolve pravastatin sodium, sodium bicarbonate, BHT, and povidone K30 in 50% ethanol solvent, add blank ball cores to the fluidization chamber, adjust the air volume, and make most of the pellets It is blown up in the fluidization chamber, and the physicochemical pressure and the flow rate of the constant flow pump are adjusted to make the drug solution atomized well, and then the drug is sprayed and applied. After the drug is applied, the hot air is used for 5 minutes to dry. After drying, continue to coat in the same way as applying the medicine, and you get it.
[0081] (2) Preparation of fenofibrate solid particles: dissolve fenofibrate, polyoxyethylene hydrogenated castor oil, and povidone K30 in 50% ethanol; mix lactose and pregelatinized starch evenly, add the liquid...
Embodiment 3
[0084] Prescription of pravastatin sodium pellets:
[0085]
[0086] Prescription of fenofibrate solid particles:
[0087]
[0088] Preparation:
[0089] (1) Preparation of pravastatin sodium pellets: pass the raw materials through a 100-mesh sieve, fully mix with microcrystalline cellulose, calcium hydrogen phosphate, and sodium bisulfite; add hypromellose 50% ethanol solution to make a soft material ; Extrude the soft material into strips through the extruder to form a smooth short branch; quickly add the extruded product to the spheronizer for spheronizing, and take it out after a certain period of time to obtain pellets; : the prepared pellets Dry at 50°C to obtain the finished drug-containing pellets. Finally, the drug-containing pellets are placed in a fluidized bed for coating.
[0090] (2) Preparation of fenofibrate solid particles: Dissolve fenofibrate, poloxamer F188, and povidone K30 in 50% ethanol; mix lactose and microcrystalline cellulose evenly, add li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com