Method for detecting pancreatic cancer
一种检测方法、胰脏癌的技术,应用在检测胰脏癌领域,能够解决不能胰脏癌病人显示出显著的差异等问题,达到快速又简单检测的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0127] Examples of the present invention are shown below to illustrate the present invention in detail. However, the present invention is not limited to the following examples.
[0128] (Preparation Example 1) Preparation of PTL
[0129] Agaricus aureus lectin (PTL) was isolated and purified from Agaricus aureus according to the purification procedure shown below.
[0130] (extraction)
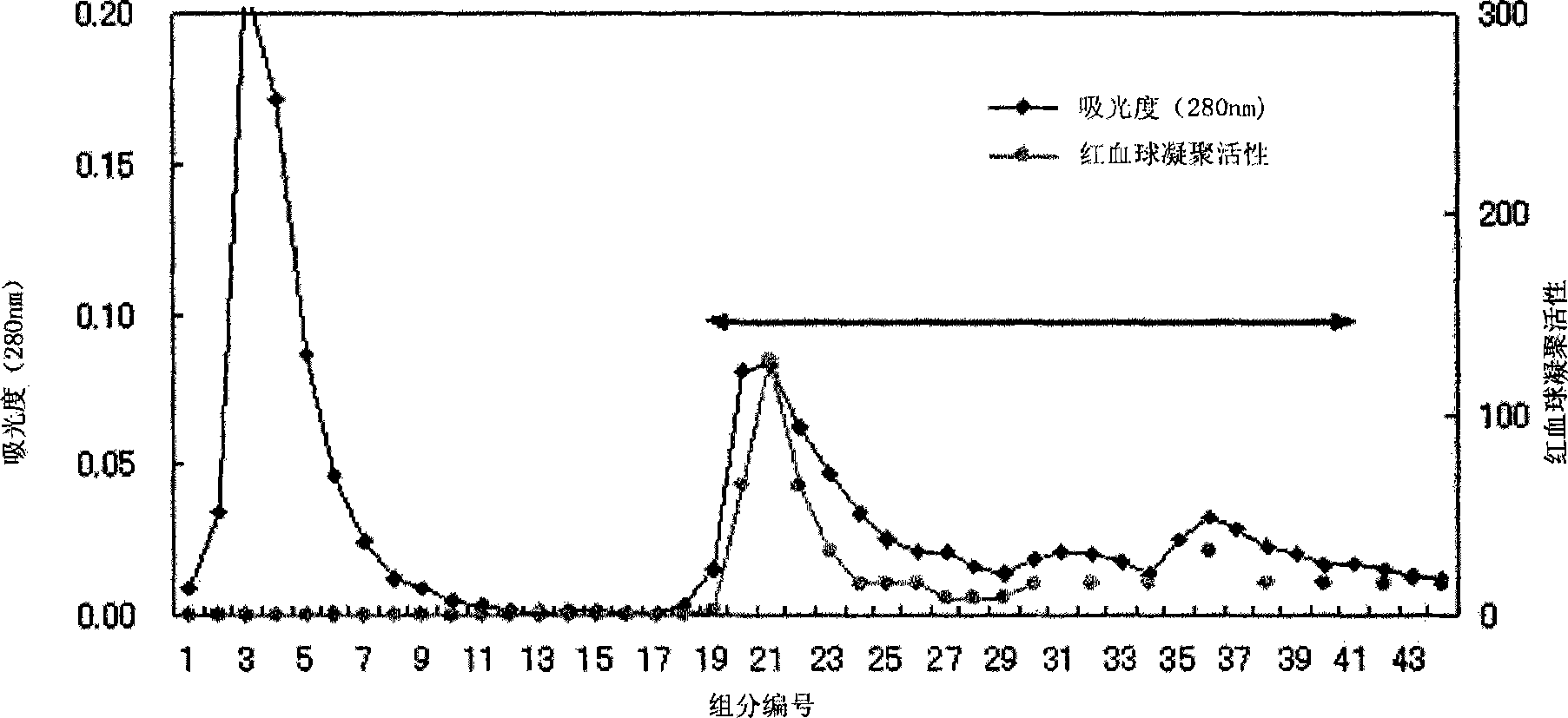
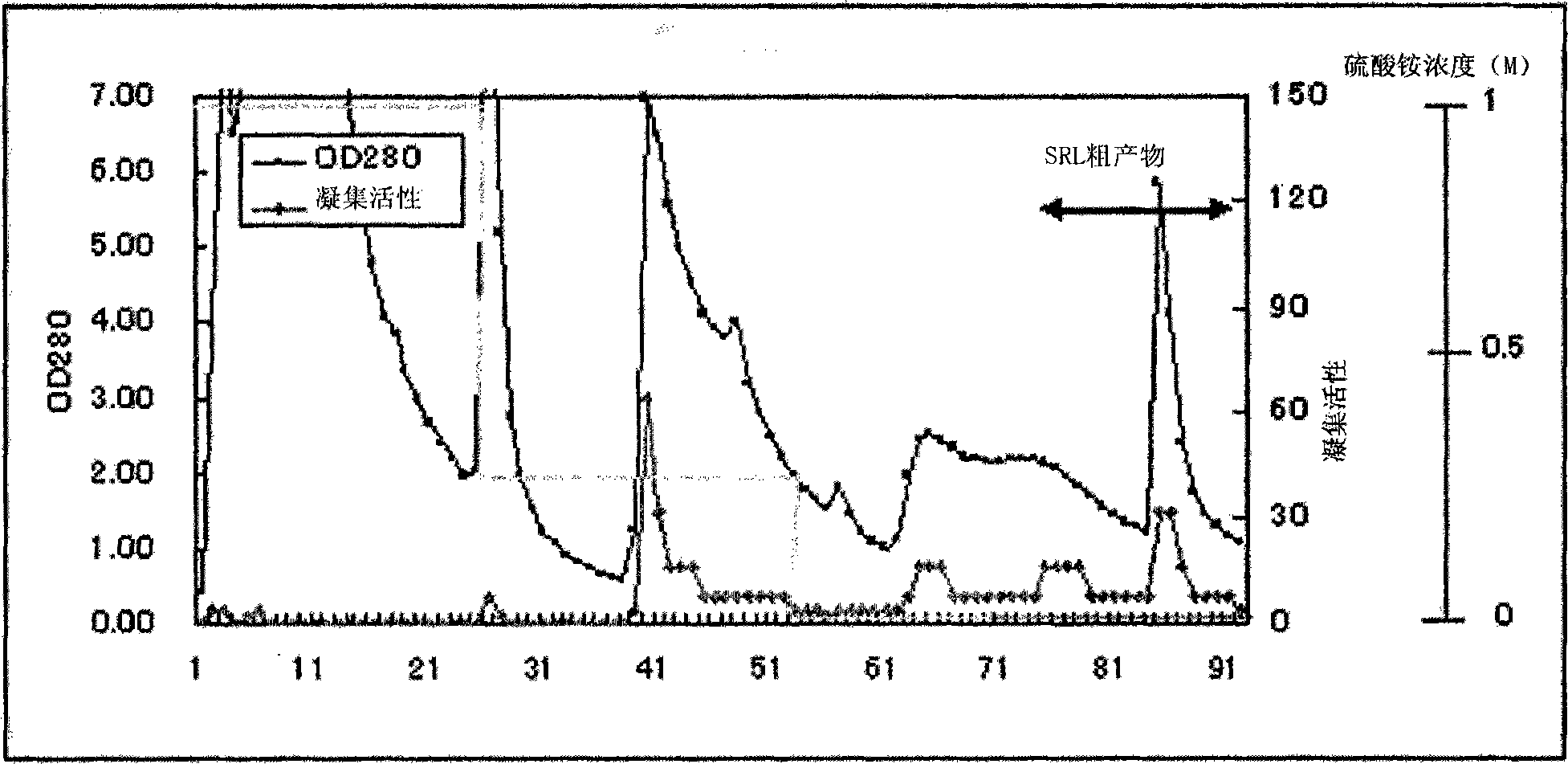
[0131] The lyophilized powder (2.5 g) obtained by lyophilizing Agaricus aureus (7.5 g) was extracted with 50 ml, 10 mM Tris buffer (pH 7.2) for 2 hours at 4°C. The obtained extract was centrifuged (15000rpm, 20min, 4°C). Then, the supernatant was filtered through gauze to obtain the first extract. The extraction residue was extracted with 50 ml of 10 mM Tris buffer (pH 7.2) overnight at 4°C. After extraction, centrifuge (15000 rpm, 20 min, 4° C.), and filter the supernatant through gauze to obtain a second extract. Then, these extracts are jointly filtered through filter paper to obtai...
reference example 1
[0146] Reference Example 1: Detection of various glycoproteins in serum by lectin.
[0147] Study by ELISA the affinity of the glycoproteins shown in Table 1 to the biotin-labeled lectins shown below by ELISA:
[0148] PTL (a trehalose α1→6 specific lectin that can be used in the invention), and
[0149] The following commercially available lectins are considered to be specific for trehalose:
[0150] LCA (manufactured by Nippon Seikagaku Co., Ltd., J-Oil Co., Ltd.),
[0151] AAL (manufactured by Nippon Biochemical Industry Co., Ltd., J-Oil Co., Ltd.),
[0152]Lotus (manufactured by Nippon Seikagaku Co., Ltd., J-Oil Co., Ltd.) and
[0153] UEA-I (manufactured by Nippon Seikagaku Co., Ltd., J-Oil Co., Ltd.).
[0154] Serum glycoprotein (human serum albumin (manufactured by CALBIOCHEM), immunoglobulin G (manufactured by Sigma), transferrin (manufactured by Sigma), fibrinogen (manufactured by AbD Serotec), immunoglobulin A (manufactured by BETYL) manufactured), α2-macroglobu...
Embodiment 1
[0167] (Example 1) Lesion haptoglobin in the culture supernatant of pancreatic cancer cell lines was detected by ELISA using PTL.
[0168] Reference Example 1 shows that PTL has no affinity for haptoglobin. Therefore, PTL detects haptoglobin extracted from the medium supernatant of pancreatic cancer cell line (PSN-1, purchased from DS PHARMA) and haptoglobin extracted from human serum as a negative control (purchased from BIODESIGN Company ) were assessed by ELISA. Similar tests to PTL were performed on LCA and AAL as positive controls.
[0169] (Extraction of haptoglobin from pancreatic cancer cells)
[0170] 1000 mL of the culture supernatant of the pancreatic cancer cell line was filtered with an ultrafilter (product name: VIVA SPIN20-10K, manufactured by SARTORIUS), and concentrated to 1 mL. The above concentrated solution was added to the gel (NHS-activated Sepharose 4 fast flow (manufactured by GE Healthcare)) in which the anti-haptoglobin antibody (manufactured by Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com