Method for preparing 9a-hydroxy androstendione
A technology for hydroxyandrostenedione and seeds, which is applied in the field of preparing 9a-hydroxyandrostenedione, can solve the problem that it is not suitable for industrial production of 9a-OHAD, the cost of the substrate androstenedione is high, and the conversion rate of androstenedione is poor. Advanced problems, to achieve the effect of shortening fermentation time, high yield and low by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Activated strain: Inoculate Mycobacterium fortuitum ATCC35855 into sterile slant medium (yeast extract powder 1wt%; beef extract 0.5wt%; glucose 0.1wt%; sodium chloride 0.9wt%; agar 2wt%; pH6.8-7.0), culture at 30°C for 4 days until the whole slope is covered with bacteria.
[0028] Expanded cultivation: Scrape a well-growing single colony with an inoculation loop, inoculate it into a 250ml Erlenmeyer flask containing 30ml sterile seed medium, and cultivate it for 24 hours at a cultivation temperature of 30°C and a rotational speed of 200rpm. The seed medium includes: based on the total mass of the seed medium, phytosterols 0.05wt%, glucose 0.1wt%, ammonium sulfate 0.2wt%, magnesium sulfate heptahydrate 0.001wt%, dipotassium hydrogen phosphate 0.1wt%, yeast powder 0.5wt%, peptone 0.5wt%, sodium chloride 0.1wt%, calcium carbonate 0.1wt%, the balance is water; and the pH of the fermentation broth is 6.5-7.5.
[0029] Fermentation culture: Inoculate 5ml of bacterial liqui...
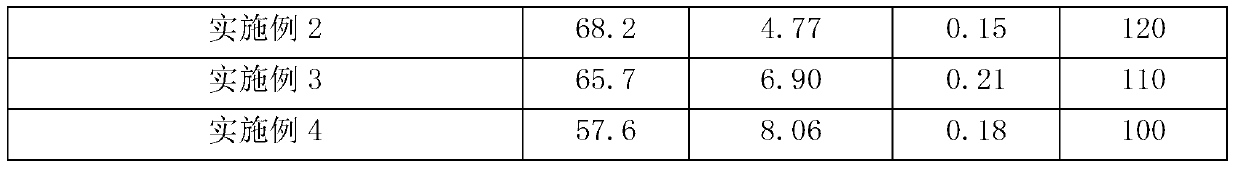
Embodiment 2
[0033] Expanded culture: insert the slant cells of Mycobacterium fortitum ATCC35855 in good growth state into a 250ml Erlenmeyer flask equipped with 30ml of sterile seed medium, and then vibrate at a culture temperature of 35°C and a rotation speed of 230rpm for 18 hours;
[0034] Fermentation culture: Then draw 5ml of seed solution according to the inoculation amount of 10% by volume and put it into a 500ml Erlenmeyer flask filled with 50ml of sterile fermentation broth, then culture at a temperature of 35°C and a rotation speed of 250rpm for 120 hours.
[0035] Then, the HPLC method described in Example 1 was used to measure the conversion rate of phytosterols, 9a-OH AD, and the concentration of by-product AD in the fermentation culture.
[0036] The seed culture medium and fermented liquid used in the present embodiment are respectively as follows:
[0037] The seed medium includes: phytosterol 0.075wt%, glucose 0.5wt%, ammonium sulfate 0.6wt%, magnesium sulfate heptahydrat...
Embodiment 3
[0041] Expanded culture: insert the slant cells of Mycobacterium fortitum ATCC35855 in a good growth state into a 250ml Erlenmeyer flask equipped with 30ml sterile seed medium, and then vibrate at a culture temperature of 32°C and a rotation speed of 230rpm for 24 hours;
[0042] Fermentation culture: Then draw 5ml of seed solution according to the inoculation amount of 10% by volume and put it into a 500ml Erlenmeyer flask filled with 50ml of sterile fermentation broth, then culture at a temperature of 32°C and a rotation speed of 250rpm for 110 hours.
[0043] Then, the HPLC method described in Example 1 was used to measure the conversion rate of phytosterols, 9a-OH AD, and the concentration of by-product AD in the fermentation culture.
[0044] The seed culture medium and fermented liquid used in the present embodiment are respectively as follows:
[0045] The seed medium includes: phytosterol 0.1wt%, glucose 0.25wt%, ammonium sulfate 0.3wt%, magnesium sulfate heptahydrate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com