PD-L1 affinity peptide with anti-tumour activity and application for same
A technology of PD-L1 and anti-tumor activity, which is applied in the application of this peptide, and the field of PD-L1 affinity peptide, can solve the problems of poor curative effect and achieve the effect of large lethality and good anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
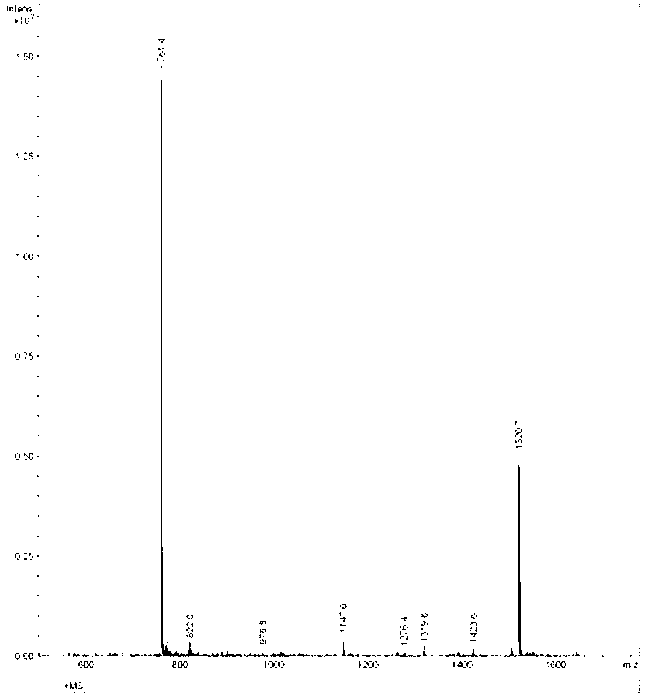
[0016] The PD-L1 affinity peptide P1 with anti-tumor activity described in the present invention is a polypeptide screened based on phage display peptide library technology. The amino acid sequence of the polypeptide P1 is: Phe-Pro-Asn-Trp-Ser-Leu- Arg-Pro-Met-Asn-Gln-Met; molecular weight 1520.7.
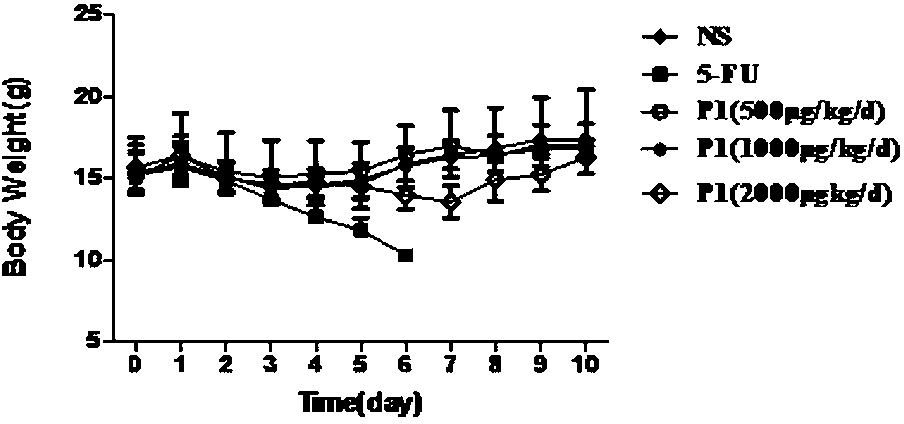
[0017] The experimental method and result involved in the present invention are as follows:
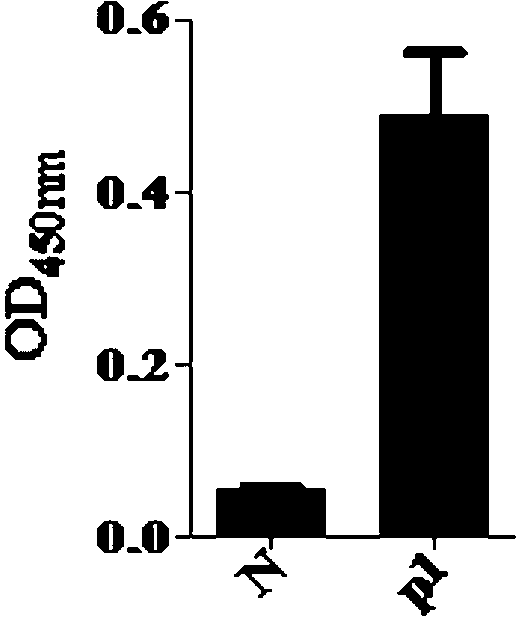
[0018] 1. ELISA identification of affinity between P1 phage monoclonal and PD-L1
[0019] The solid-phase screening method was used to screen the phage display 12-peptide library. After 5 rounds of screening, phage monoclonals with affinity to the extracellular domain of the target protein PD-L1 were enriched round by round, and the recovery rate increased by about 1000 times. Then 50 monoclonal phage coeruleus were randomly selected from the plates of the 4th and 5th rounds of screening, and their affinity was identified by ELISA. Among them, 17 were obviously positive, and the positiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com